Assessment of herbicide-induced changes in beneficial microflora of Bajra (Pennisetum glaucum L.) rhizosphere
Abstract
The present study investigates the impact of various herbicides on beneficial microbial populations in the rhizosphere of Pennisetum glaucum (Bajra), focusing on Azotobacter, Azospirillum, and phosphate-solubilizing bacteria (PSB). Herbicidal treatments significantly influenced microbial populations at different crop growth stages, with a peak observed at 60 days after sowing (DAS). Among pre-emergence herbicides, Pendimethalin consistently supported the highest populations of all three microbial groups, while Oxyfluorfen exhibited suppressive effects. Phenaxoprop-ethyl emerged as the most favorable post-emergence herbicide, enhancing microbial proliferation, whereas Imazethapyr consistently reduced microbial abundance. Sequential application of Pendimethalin + Propaquizafop-ethyl maintained the highest microbial populations, while the combination with Imazethapyr was the least favorable. Microbial populations declined slightly by harvest, though treatment trends remained consistent. The findings highlight Pendimethalin and Phenaxoprop-ethyl as microbiologically compatible options, while Imazethapyr poses potential risks to rhizospheric health. These results emphasize the importance of selecting herbicides not only for weed management efficacy but also for their compatibility with beneficial soil microflora crucial for nutrient cycling and plant productivity. The study underscores the ecological benefits of integrating herbicide strategies with microbial sustainability, recommending Pendimethalin + Propaquizafop-ethyl for promoting rhizospheric microbial health in sustainable bajra cultivation.
Introduction
Bajra (Pennisetum glaucum L.), commonly known as Pearl Millet, is an essential cereal crop grown primarily in the arid and semi-arid regions of Africa and Asia. This resilient crop is known for its ability to thrive in harsh environmental conditions such as low rainfall, high temperatures, and poor soils, making it a vital food source for millions of people in these areas. Bajra is highly nutritious, containing carbohydrates (67%), protein (11.6%), and minerals (2.7%), which make it an important dietary staple for both humans and livestock (Tejagouda, 2012). It is particularly significant in regions with limited water resources, where its drought-tolerant nature ensures food security and sustenance. However, despite its hardiness, Bajra productivity is often constrained by factors such as weed competition, which can substantially reduce crop yield.
Weed control in Bajra cultivation is commonly achieved through the use of herbicides. Herbicides offer a convenient and effective means of managing weed populations, which are known to reduce yields by up to 50% in some regions (Dobrovoljskiy and Grishina, 1985). However, the increased reliance on herbicides in modern agriculture raises concerns regarding their potential impact on soil health and microbial communities. Herbicides are designed to target and control weeds, but their application may also affect non-target organisms, particularly the beneficial microorganisms present in the soil. Soil microorganisms, including nitrogen-fixing bacteria, mycorrhizal fungi, and other beneficial microflora, play a crucial role in nutrient cycling, organic matter decomposition, and overall plant health. These microorganisms contribute to plant growth by enhancing nutrient uptake, suppressing soilborne pathogens, and improving soil structure. Any disturbance in their populations due to herbicide application can adversely affect soil fertility, plant growth, and crop yield (Ayansina et al., 2003).
The residual effects of herbicides on soil microflora, particularly beneficial microorganisms, remain an area of concern in agricultural research. Some herbicides, especially those with low biodegradability, can persist in the soil for extended periods, leading to long-term alterations in microbial diversity and activity. These herbicide residues may interfere with the natural soil processes, resulting in reduced microbial populations or shifts in microbial community composition. Such changes can negatively influence soil health, nutrient cycling, and the availability of essential nutrients for plants (Ashim et al., 2008). Furthermore, herbicides may disrupt the balance between beneficial and harmful microorganisms, potentially leading to the dominance of pathogenic microbes that can further hinder plant growth (Amakiri, 1982).
The present study focuses on the impact of herbicides on beneficial soil microflora in the rhizosphere of Bajra. Specifically, it aims to evaluate how residual herbicide toxicity affects the populations of beneficial microorganisms that are critical for soil fertility and plant health. By investigating the effects of herbicides on the microbial diversity and activity in the Bajra rhizosphere, the study seeks to contribute to a better understanding of the long-term consequences of herbicide use on soil microbial communities. The findings will provide valuable insights into how herbicide-induced changes in soil microflora can affect Bajra growth, nutrient availability, and crop productivity. Ultimately, this research aims to support the development of more sustainable herbicide management practices that minimize harm to beneficial microorganisms and enhance overall soil health, ensuring the continued productivity of Bajra in arid and semi-arid regions (Hutsch, 2001).
Material and Methods
Study site and experimental design
A field experiment was conducted to investigate the residual effects of herbicides on soil microbial communities, including beneficial microflora, and their subsequent influence on the growth and yield of Pennisetum glaucum L. (Bajra) under rainfed conditions during the rainy season. The experimental site was part of a long-term cropping sequence, wherein various herbicidal treatments were applied to a preceding chickpea (Cicer arietinum L.) crop. The objective was to assess the residual impact of these herbicides on the rhizospheric soil microbiota and subsequent crop performance.
The study employed a randomized complete block design (RCBD) comprising 18 treatments and three replications. Treatments included both pre-emergence and post-emergence herbicides applied to the chickpea crop. Pre-emergence herbicides were administered on the day of sowing, whereas post-emergence herbicides were applied 20 days after sowing (DAS).
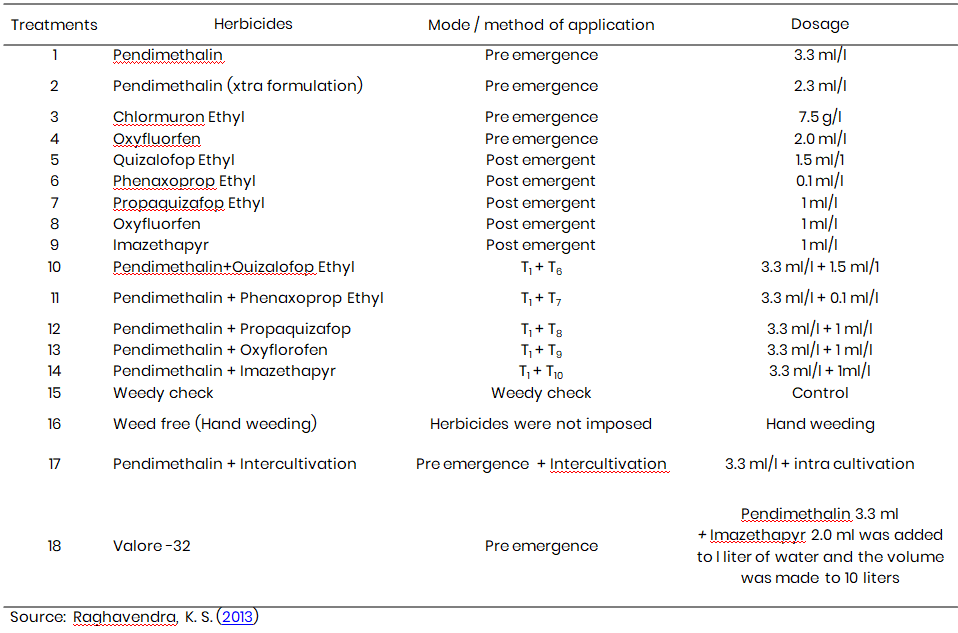
Each plot measured 3 m × 4 m, with row spacing maintained at 45 cm and intra-row spacing at 15 cm. The hybrid variety ICTP-8230 of Bajra was sown in all experimental plots following standard agronomic practices. The treatment details consisting of mode of application and dosage are provided in Table 1.
Herbicide Treatments
Herbicidal treatments included both individual and combined formulations to simulate practical field conditions. Table 1 summarizes the herbicides employed, their application stages (pre- or post-emergence), and dosages. These treatments were selected to reflect real-world usage patterns and to assess the persistence and subsequent effects of herbicidal residues in soil.
Soil sampling and physicochemical analysis
Soil samples were collected at four time points: before sowing of Bajra, and at 20, 45, and 60 DAS, as well as at crop harvest. Samples were obtained from the rhizosphere zone at a depth of 0–15 cm using a soil auger, composited, and processed for analysis.
Soil pH was determined in a 1:2.5 soil-to-water suspension using a glass electrode pH meter. Electrical conductivity (EC) was measured using a conductivity bridge, following the protocol outlined by Jackson (1967).
Enumeration of soil microbial populations
Total soil microbial populations, including key beneficial microorganisms such as Azotobacter, Azospirillum, and phosphate solubilizing microorganisms (PSM), were assessed using the standard serial dilution and plating technique on selective culture media. General bacterial populations were enumerated on nutrient agar, while specific microbial groups were isolated and quantified using their respective selective media. Azotobacter was cultured on Waksman No. 77 medium (Allen, 1953), Azospirillum was grown on nitrogen-free malate medium, and PSM were isolated using Pikovskaya’s agar medium (Bunt Rovira, 1955). After inoculation, plates were incubated under optimal laboratory conditions, and microbial colonies were counted and expressed as colony-forming units (CFU) per gram of dry soil.
Statistical analysis
All collected data, including microbial counts and soil chemical parameters, were subjected to analysis of variance (ANOVA) using the statistical procedure described by Gomez and Gomez (1984). The F-test was applied to determine the significance of treatment effects at a probability level of P ≤ 0.05. Wherever applicable, treatment means were compared using the least significant difference (LSD) test to determine statistically significant differences among treatments.
Table 1: Treatments imposed on previous chickpea crop using different herbicides
Results and Discussion
Effect of herbicides on Azotobacter population in Bajra rhizosphere
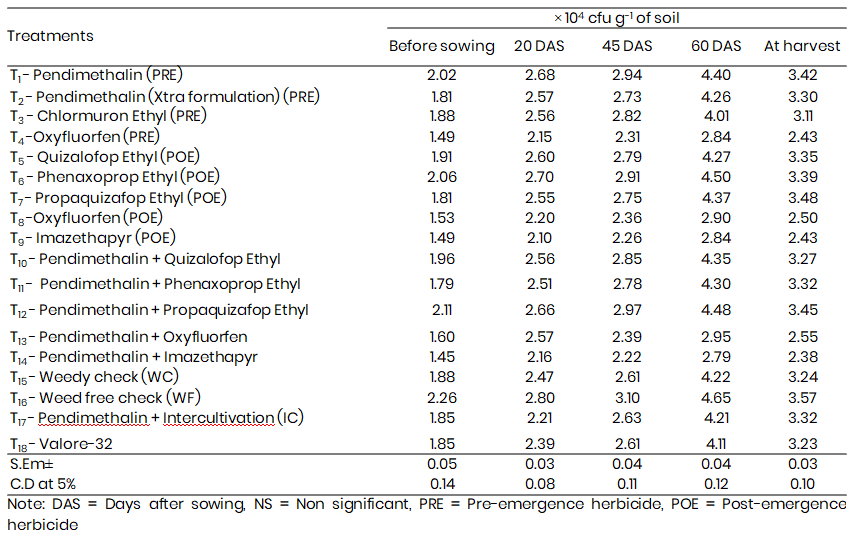
The application of herbicides significantly affected Azotobacter populations in the rhizospheric soil of Pennisetum glaucum across various growth stages (Table 2). Across all treatments, the population of Azotobacter consistently peaked at 60 days after sowing (DAS), suggesting enhanced microbial activity during this phase.
Among pre-emergence herbicides, Pendimethalin consistently supported the highest Azotobacter populations across all stages, with a maximum of 4.40 × 10⁴ cfu g⁻¹ soil at 60 DAS, whereas Oxyfluorfen showed the most suppressive effects (2.84 × 10⁴ cfu g⁻¹). Post-emergence treatments followed a similar trend: Phenaxoprop-ethyl favored microbial proliferation (4.50 × 10⁴ cfu g⁻¹), while Imazethapyr exhibited inhibitory effects (2.84 × 10⁴ cfu g⁻¹). Sequential applications of Pendimethalin + Propaquizafop-ethyl consistently maintained higher microbial counts, peaking at 4.48 × 10⁴ cfu g⁻¹ at 60 DAS. Conversely, Pendimethalin + Imazethapyr reduced the population to 2.79 × 10⁴ cfu g⁻¹.
Following 60 DAS, a decline in population was observed at harvest. However, the relative trends persisted, with Pendimethalin and Phenaxoprop-ethyl continuing to sustain the highest Azotobacter counts and Imazethapyr remaining the most inhibitory.
Table 2: Residual effect of herbicides on Azotobacter population at different growth stages of Bajra
Effect of herbicides on Azospirillum population in Bajra rhizosphere
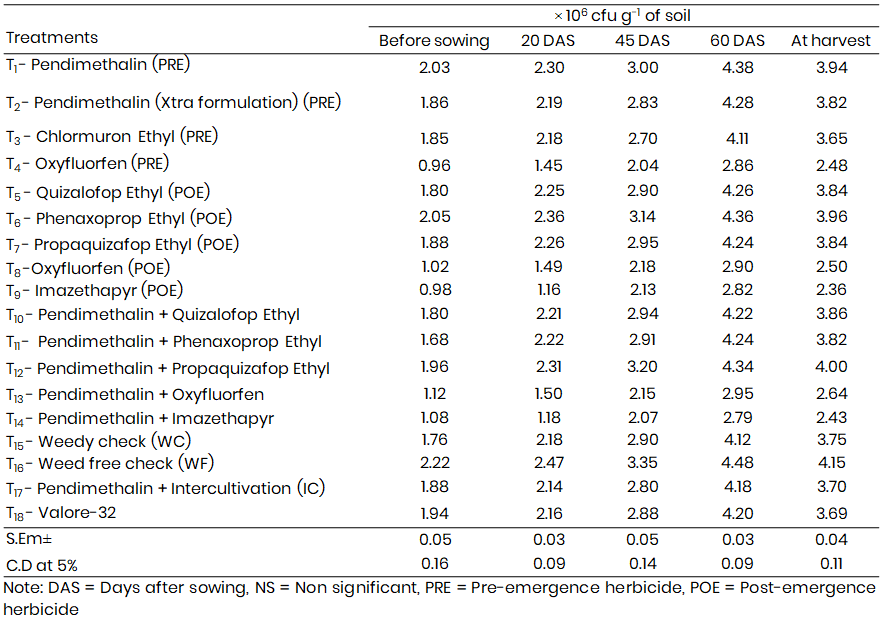
Azospirillum populations also demonstrated significant sensitivity to herbicidal influence (Table 3). Population densities peaked at 60 DAS across all treatments, indicating this stage as optimal for Azospirillum proliferation, likely due to heightened root exudation and favorable soil conditions.
Pendimethalin once again emerged as the most supportive pre-emergence herbicide, with the Azospirillum population reaching 4.38 × 10⁶ cfu g⁻¹ soil at 60 DAS. Oxyfluorfen, in contrast, suppressed microbial abundance (2.86 × 10⁶ cfu g⁻¹). Among post-emergence herbicides, Phenaxoprop-ethyl resulted in the highest count (4.36 × 10⁶ cfu g⁻¹), while Imazethapyr was consistently detrimental (2.82 × 10⁶ cfu g⁻¹).
Sequential application of Pendimethalin + Propaquizafop-ethyl supported the highest population (4.34 × 10⁶ cfu g⁻¹), while Pendimethalin + Imazethapyr was the least favorable (2.79 × 10⁶ cfu g⁻¹).
At harvest, populations declined slightly, in line with senescing roots and declining nutrient levels. However, relative treatment efficacy remained constant, reaffirming the safety of Pendimethalin and Phenaxoprop-ethyl.
Table 3: Residual effect of herbicides on Azospirillum population at different growth stages of Bajra
Effect of herbicides on phosphate solubilizing bacteria (PSB) in Bajra rhizosphere
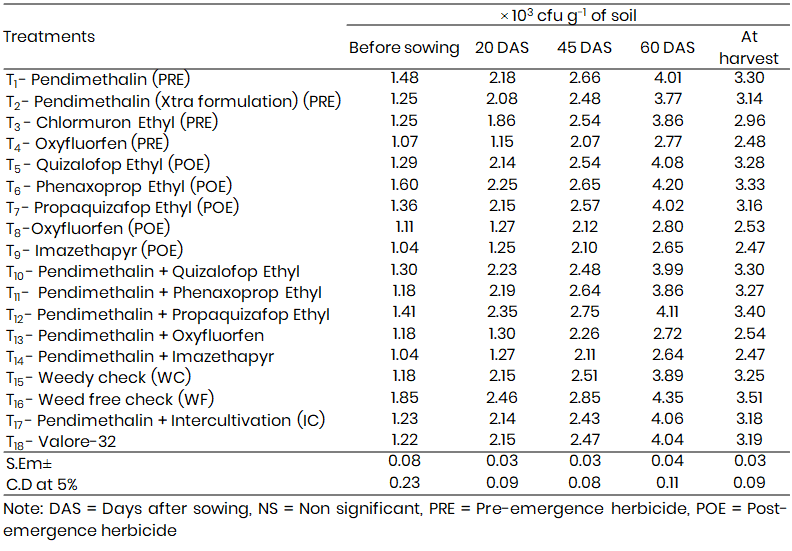
Phosphate solubilizing bacteria (PSB) populations followed a similar trend (Table 4), with maximum proliferation at 60 DAS. Among pre-emergence herbicides, Pendimethalin again yielded the highest population (4.01 × 10³ cfu g⁻¹), while Oxyfluorfen was least favorable (2.77 × 10³ cfu g⁻¹). Phenaxoprop-ethyl facilitated the highest PSB counts among post-emergence herbicides (4.20 × 10³ cfu g⁻¹), whereas Imazethapyr markedly inhibited PSB (2.65 × 10³ cfu g⁻¹). Sequentially, Pendimethalin + Propaquizafop-ethyl promoted the highest PSB count (4.11 × 10³ cfu g⁻¹), whereas Pendimethalin + Imazethapyr had a suppressive effect (2.64 × 10³ cfu g⁻¹).
At harvest, a reduction in microbial activity was evident, yet the relative performance of the treatments remained consistent, emphasizing the persistent effects of herbicide combinations on microbial ecology.
The results clearly indicate that herbicide applications substantially influence beneficial microflora in the rhizosphere of Pennisetum glaucum, with Pendimethalin and Phenaxoprop-ethyl showing minimal to no adverse effects on Azotobacter, Azospirillum, and PSB populations. In contrast, Oxyfluorfen and particularly Imazethapyr consistently demonstrated microbial suppressiveness, regardless of timing or combination. This variation in microbial response aligns with previous reports that certain herbicides, depending on their chemical nature, persistence, and mode of action, can selectively inhibit or promote microbial populations (Cycoń and Piotrowska-Seget, 2015; Walia et al., 2017). The superior microbial performance at 60 DAS across all treatments is likely attributed to increased root biomass, exudation, and active rhizospheric interactions (Mishra et al., 2013).
Importantly, the sequential combination of Pendimethalin + Propaquizafop-ethyl emerged as the most microbiologically favorable treatment, supporting high populations across all three microbial groups, thus suggesting its compatibility with sustainable crop management strategies. In contrast, the Pendimethalin + Imazethapyr combination consistently recorded the lowest microbial counts, raising concerns about its long-term impact on soil health. These findings underscore the necessity of selecting herbicides not solely based on weed control efficacy, but also on their ecological compatibility with soil microbial communities vital to nutrient cycling and plant growth promotion.
Table 4: Residual effect of herbicides on phosphate solubilizing bacteria (PSB) population at different growth stages of Bajra
Conflicts of interests
Authors declare that there is no conflict of interest exists.
References
Allen, O. N. (1953). Waksman No. 77 medium for isolation of Azotobacter. Soil Science, 75(3), 123–128. https://doi.org/10.1097/00010694-195303000-00003
Amakiri, S. O. (1982). Impact of herbicides on soil microorganisms and crop productivity. Journal of Environmental Science and Technology, 15(2), 233–240.
Ashim, N. S., Kumar, S., & Patel, S. K. (2008). Herbicide residues and their effect on soil microbial diversity and activity. Environmental Monitoring and Assessment, 147(1–3), 205–212.
Ayansina, A. D., Olasunkanmi, L. O., & Akinyemi, O. O. (2003). Fate of herbicides in soil: Impact on groundwater contamination and soil microflora. Journal of Environmental Sciences, 40(5), 574–580.
Bunt, J. S., & Rovira, A. D. (1955). Microbial flora and methods for enumeration in soil. Journal of Applied Microbiology, 22(1), 78–89. https://doi.org/10.1111/j.1365-2672.1955.tb04385.x
Cycoń, M., & Piotrowska-Seget, Z. (2015). Biochemical and microbial soil functioning after application of fungicides, herbicides and insecticides: A review. Soil Biology and Biochemistry, 75, 54–64. https://doi.org/10.1016/j.soilbio.2014.10.015
Dobrovoljskiy, A. O., & Grishina, T. A. (1985). Effect of herbicide application on weeds and soil microorganisms in agriculture. Soil Science and Agrochemistry, 42(7), 168–174.
Gomez, K. A., & Gomez, A. A. (1984). Statistical procedures for agricultural research (2nd ed.). John Wiley & Sons.
Hutsch, B. W. (2001). Herbicide effects on soil functions and microbial activity: Implications for agricultural sustainability. Soil Biology and Biochemistry, 33(3), 407–417.
Jackson, M. L. (1967). Soil chemical analysis. Prentice Hall.
Mishra, S., Nautiyal, C. S., & Sharma, A. (2013). Rhizospheric microbial communities and their role in soil health and plant growth under herbicidal stress. Journal of Applied Microbiology, 115(5), 1145–1160. https://doi.org/10.1111/jam.12309
Raghavendra, K. S. (2013). Effect of pre and post emergence herbicides on rhizospheric microflora, nodulation, growth and yield of chickpea (M.Sc. (Agri.) thesis). University of Agricultural Sciences, Raichur.
Tejagouda, M. (2012). Agricultural significance and growth characteristics of bajra (Pennisetum glaucum L.) in dry regions. Journal of Agricultural Research, 58(3), 113–120.
Walia, A., Mehta, P., Guleria, S., Chauhan, A., & Shirkot, C. K. (2017). Effect of herbicides on soil microbial population and enzymatic activity. Environmental Monitoring and Assessment, 189(5), 219. https://doi.org/10.1007/s10661-017-5901-9
Copyright
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.

