Assessment of pesticide residues level in surface and groundwater from Melleri irrigation farmlands in Gombe state, Nigeria
Abstract
This study determined pesticide residues levels in surface and groundwater from irrigation farmlands in the Kwami Local Government Area of Gombe State, Nigeria. Pesticide residues were analyzed using a gas chromatograph equipped with a 63Ni electron capture detector (SHIMADZU GC-2010). The mean concentration of aldrin in the surface water samples ranged from 0.0001 to 0.0013 mg kg-¹ and 0.0024 to 0.0041 mg kg-¹ in groundwater, whereas atrazine was below the detectable limit in all the studied samples. Chlorpyrifos ranged from 0.00 to 0.015 mg kg⁻¹ in both samples, cyhalothrin was below the detectable limit, dichlorvos ranged from 0.00 to 0.031 mg kg⁻¹, while endosulfan ranged from 0.0034 to 0.086 mg kg⁻¹ in the soil samples. The levels of cyhalothrin ranged from 0.00 to 0.0094 mg kg⁻¹ in groundwater and 0.0013 to 0.0032 mg kg⁻¹ in surface water, whereas those of endrin, heptachlor, imidacloprid, lambda-cyhalothrin, and Lindane were below the detectable limit. The levels of these pesticide residues may accumulate in food crops through the uptake of water. However, monitoring and stringent regulations should be imposed on the use of pesticides in soil and foodstuff for public health protection.
Introduction
The widespread use of pesticides for agricultural and non-agricultural purposes has resulted in the presence of residues in surface and ground water resources (András et al., 2015). Contamination occurs not only due to the current use of agrochemicals but also due to the leaching of persistent ingredients from soil. Pesticide contamination of surface water in a particular region depends on several factors, such as the proximity of crop fields to surface water, characteristics of surrounding fields (soil, grassland, slope, and distance to water bodies), and climate conditions (temperature, humidity, wind, and precipitation) (Judith, 2022). Consequently, pesticide residues are being reported as common organic contaminants worldwide in surface waters and other environmental matrices (András et al., 2015). The physicochemical properties of pesticide compounds, particularly their solubility in water and organic solvents, characterized by their octanol-water partition coefficients, determine theirleaching characteristic of into surface and ground waters (Pérez-Lucas et al., 2019).
Groundwater, like any other water resource, is not only of public health and economic value; it also has an important ecological function (Sulaiman et al., 2016). The contamination of groundwater resources by pesticides has raised environmental concern (Pérez-Lucas et al., 2019). The problem has become more prominent in countries where groundwater aquifers constitute the main drinking water resources for rural and adjacent urban areas (Carrard et al., 2019). Pesticide residues reach the aquatic environment through direct runoff, leaching, careless disposal of empty containers, equipment washings, etc. (El-Saeid et al., 2011). Widespread use of pesticides could lead to extensive pollution of the environment and constitute a potential and/or deliberate risk to human health because some of pesticides are classified as a probable human carcinogen (Mohamed and Ahmed, 2020).
Surface water in agricultural areas can be contaminated by pesticide residues with possible adverse effects on the ecosystem (Lundqvist et al., 2019). Surface water contamination may have eco-toxicological effects on aquatic flora and fauna as well as for human health if used for public consumption (Bashir et al., 2020). Environmental monitoring of pesticide residues in water is generally based on chemical analysis of the pesticides and known metabolites or degradation products thereof (Lundqvist et al., 2019). Leaching is a form of environmental pollution in which chemicals drain away from the treated region to non-targeted environments (Pérez-Lucas et al., 2018). In this way, surface waters have the potential of becoming contaminated when irrigation water that has passed over pesticide-treated plants and/or the environment drains or leaches into the surface waters (Syafrudin et al., 2021). Another source of pollution is drift, which occurs if a pesticide sprays targets, having been deflected by the wind or resulting from the error of missing the intended target, thereby landing on a non-targeted farm area (Damalas and Eleftherohorinos, 2011). In view of this, the present study aimed to determine pesticide residues levels in water samples collected from different farmlands in Malleri, Kwani local government area of Gombe state, Nigeria.
Material and Methods
Area of the study
The Malleri is a village in the Kwami local government area, and Kwami LGA is one of the eleven Local Governments of Gombe State. It is located about 6 kilometers in the northeastern part of Gombe town and lies on the latitude 10°29'35''N and longitude 11°12'36''E (Fig. 1). The area falls within the northern Guinea savanna zone of the Kwami Local Government Area of Gombe State. Kwami has a total area of 1,787 km² (690 sq mi). It has a population of 195,298 (2006, Census). The area receives an average annual rainfall of approximately 800 mm, which is sufficient for a single farming season. The annual rainfall pattern is erratic at the beginning of the rainy season, starting in April, and intensifying as the season advances, rising from 600 to 1000 mm. The maximum temperature could rise as high as 41°C and the minimum as low as 16°C. The temperatures were usually high, with an average of 34°C.
Fig. 1: Map of the study area
Sampling techniques
The water samples were collected using the grab sampling method. Samples were collected in 1-L amber-colored glass bottles. Sampling bottles were rinsed with water and carefully filled to overflow without trapping air bubbles in the sealed bottles. The samples were transported in a cool box containing ice packs. The containers were prepared by washing with detergent, rinsing with tap water, ultrapure water (Millipore), and air-drying. After transportation to the laboratory, samples were stored at 4°C, and extraction was performed within 48 h.
Chemicals and Reagents
The solvents used for the extraction were obtained from Merck (HPLC grade for chromatography). Individual pesticide stock standard solutions were prepared by exact high-purity substances in 10 mL volumetric flasks and filled with an appropriate solvent such as acetone and n-hexane. All stock standard solutions were stored in a deep freezer protected from light at 20°C. An intermediate and working standard of suitable concentration was made from the stock as and when required.
Sample Extractions and Analysis
Water samples were filtered using Whatman No. 1 filter paper to remove debris. 800 mL of water sample was transferred into a 1-L glass-separating funnel. Then, 80 g of NaCl was added to produce a salt-out effect. The mixture is thoroughly mixed by inverting the flask three to four times. The sample was extracted three times with 160 mL of dichloromethane (80:40:40) and shaken for 3-4 min each time with periodic venting. The combined organic phase is dried by passing it through anhydrous Na2SO4. The organic phase is concentrated to 3-5 mL in a vacuum rotary evaporator (Heidolph) and further dried under a gentle stream of nitrogen in a Turbovap (Caliper Science) low-volume concentrator. The sample was reconstituted in 1 mL of n-hexane, and 1 μL of the aliquot was analyzed by GC-MS (Gas Chromatography-Mass Spectroscopy).
Sample Analysis
The pesticide residues were analyzed using gas chromatograph equipped with a 63Ni electron capture detector (SHIMADZU GC-2010), and the presence of pesticides was confirmed using a Varian Saturn 2200 GC-MS. The determination of pesticide residue had been performed following the U.S. Environmental Protection Agency (USEPA) Method 8081 B and a self-modified laboratory method using GC-SHIMADZU with an electron capture detector. A Varian Saturn 2200 gas chromatograph mass spectrometer was used to confirmthe pesticide analysis. The injection port temperature was set to 250°C, and a liner with a plug of glass wool was installed. An amount of 1 μL of the concentrated extracts was injected in split mode (1:5). Helium was used as the carrier gas at a flow rate of 0.94 mL/min. The pesticides will be separated with a 50.10 min oven temperature program as follows: initial temperature 40°C (hold 2 min), increase at 25°C min1 to 130°C (hold 0 min), increase at 12°C min1 to 180°C (hold 0 min), and finally increase at 3°C min1 to 280°C (hold 7 min). The mass spectrometer is operated in the electron impact (70 eV) selected ion monitoring (SIM) mode. The temperature of the injector and interface was 200°C and 250°C, respectively (Summaiya et al., 2014).
Data Analysis
The data obtained will be subjected to simple descriptive statistics (mean and standard deviation) using SPSS software version 25 for Windows. The mean values obtained in this study were compared with WHO standards.
Results and Discussion
Percentage of pesticide in surface and ground water samples
The percentage of pesticide residues is presented in Fig. 2. Aldrin, chlorpyrifos, cypermethrin, and endosulfan were detected in the water samples. The percentage of Aldrin in the water samples was in the range of 15.96%, 33.33%, and 23.16%, and 17.11%, 31.43%, and 2.70 in locations 1, 2, 3, and 4 for surface and ground water, respectively, while the percentage of chlorpyrifos in the water samples was 25.35% and 27.63%, and 32.20% and 32.43 in locations 1 and 3 for surface and ground water, respectively, and below the detectable limit in location 2. The percentage levels of cypermethrin in the water samples were 19.44% and 31.43% and 8.47% and 27.03% at locations 2 and 3 for surface and groundwater, respectively, and were below the detectable limit at GW1. The levels of dichlorvos in the water samples were in the order of 14.55% and 13.169% in location 1 for surface and groundwater, respectively, and below the detectable in locations 2 and 3, while the percentage levels of endosulfan in the water samples were in the range of 44.13%, 47.22%, and 36.16%, and 42.11%, 37.14%, and 37.84 in both locations 1, 2, and 3 for surface and groundwater, respectively.
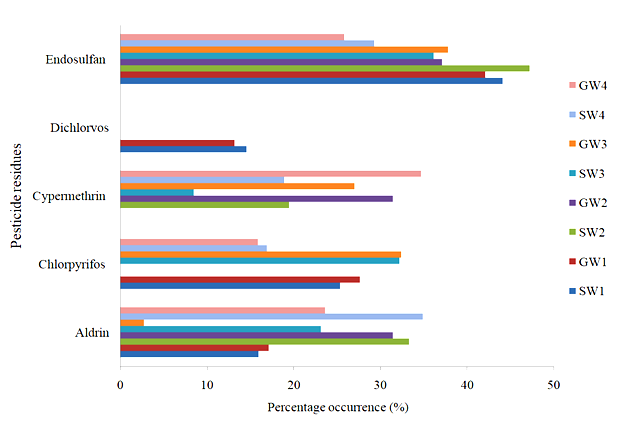
Fig. 2: Percentage (%) of pesticide residues in surface and groundwater samples
Levels of pesticide in ground and surface water samples
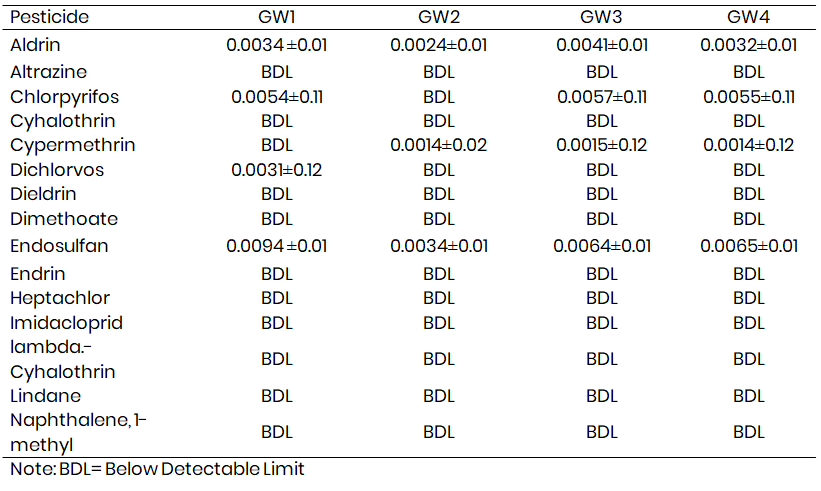
Table 1a and 1b presents pesticide levels in surface and groundwater samples from Melleri irrigation farmlands. The concentrations of altrazine, dieldrin, dimethoate, endosulfan, endrin, heptachlor, imidacloprid, lindane, naphthalene, 1-methyl, p,p'-DDT, paraquat dichloride, phenanthrene, pyrazophos, and quintozene in the samples were below the detectable limit. Aldrin concentrations in the samples ranged from 0.0013-0.0034 mg L-1, 0.0011-0.0024 mg L-1, and 0.0001-0.0041 mg L-1 at locations 1, 2, 3, and 4, respectively, while the concentrations of chlorpyrifos in the samples ranged from 0.0021-0.0054 mg L-1 and 0.0012-0.0057 mg L-1 at locations 1, 3 and 4, respectively, and were below the detectable limit at location 2 in both surface and ground water samples. The dichlorvos concentration ranged from 0.0031 to 0.03 mg L-1 at location 1 in both ground and surface water and was below the detectable limit at locations 2 and 3 for both surface and ground water samples. The endosulfan concentrations in the samples ranged from 0.0032-0.0094 mg L-1, 0.0013-0.0034 mg L-1, 0.0014-0.0064 mg L-1, and 0.0014-0.0065mg L-1 at locations 1, 2, 3, and 4 respectively for surface and groundwater.
The level of aldrin obtained in this study was below (0.015 mg L-1) that reported in a water sample from Southwestern Kenya (Nyaundi et al., 2019). Adeleye et al. (2019) reported the detection of aldrin (0.509 mg L-1) in surface and ground water (0.391 mg L-1) above the values obtained in this study. Ibrahim et al. (2019) also reported that the level of aldrin detected was higher than that obtained in this study.
Chlorpyrifos is a well-known broad-spectrum organophosphate pesticide commonly used in rural agricultural farmlands in Nigeria, and it is produced in different brands (Ononamadu et al., 2017). Ononamadu et al. (2017) reported that the content of chlorpyrifos was 0.0020-0.027 mg L-1, which was below the value of 0.0057 mg L-1 obtained in this study. The chlorpyrifos concentration detected in this study was below the European Union maximum residue level (EU MRL).
Dichlorvos, an organophosphate, is a predominant pesticide used for domestic insect control in developing countries. Acute and prolonged exposure may lead to death and genotoxic, neurological, reproductive, carcinogenic, immunological, hepatic, renal, respiratory, metabolic, dermal, and other systemic effects. Its toxicity is due to its ability to inhibit acetylcholine esterase at the cholinergic junctions of the nervous system (Okoroiwu and AIwara, 2018). The results of this were similar to those reported by Yao et al. (2023). The detected dichlorvos levels exceeded the MRL recommended by the European Union. This could be due to unawareness and misuse of pesticides use in Nigeria (Sulaiman et al., 2021).
The level of endosulfan obtained in this study was below (0.043 mg L-1) that reported in a water sample from Southwestern Kenya (Nyaundi et al., 2019). Ibrahim et al. (2019) also reported that the endosulfan content was higher than that obtained in this study.
Table 1a: Levels of pesticide residues in surface water (mg L-1) samples
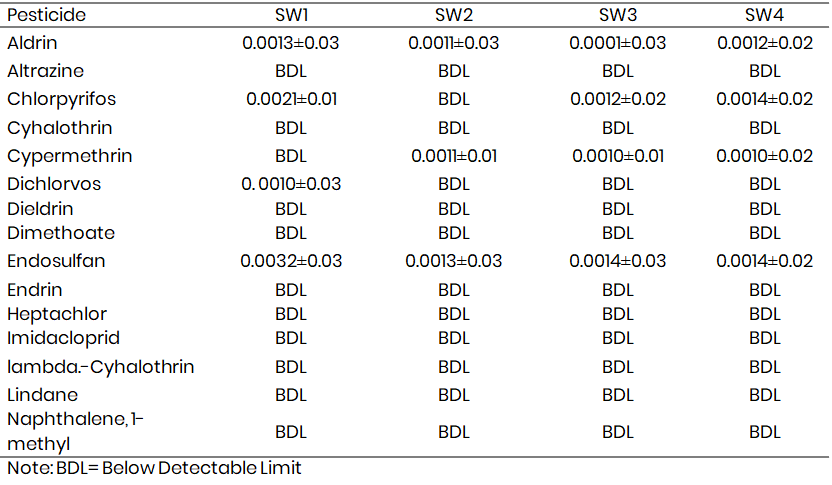
Table 1b: Levels of pesticide residues in Ground water (mg L-1) samples
Conclusion
The present study was conducted to determine pesticide residues levels in surface and groundwater cultivated with vegetables in Maleri, irrigation farmlands, Kwami Local Government Area of Gombe State, Nigeria. The water samples collected and analyzed in this study contained considerable amounts of Aldrin, Chlorpyrifos, Cypermethrin, Dichlorvos, and Endosulfan at different concentrations. In addition to aldrin, chlorpyrifos, cypermethrin, dichlorvos, and endosulfan in the sample, altrazine, cyhalothrin, dieldrin, dimethoate, endosulfan, endrin, heptachlor, imidacloprid, and lambda-cyhalothrin, lindane, naphthalene, 1-methyl, p,p'-DDT, paraquat dichloride, phenanthrene, pyrazophos, and quintozene were below the detectable limit in the studied samples. However, the need for regular monitoring of pesticide residues should be encouraged, because an increase in misuse of pesticides in agricultural produce is still ongoing in Nigeria.
References
András, S., Mária, M., & Béla, D. (2015). Monitoring pesticide residues in surface and ground water in Hungary: Surveys in 1990–2015. Journal of Chemistry. https://doi.org/10.1155/ 2015/717948
Adeleye, A. O., Sosan, M. B., & Oyekunle, J. A. O. (2019). Dietary exposure assessment of organochlorine pesticides in two commonly grown leafy vegetables in Southwestern Nigeria. Heliyon, 5, e01895.
Bashir, I., Lone, F. A., Bhat, R. A., Mir, S. A., Dar, Z. A., & Dar, S. A. (2020). Concerns and threats of contamination on aquatic ecosystems. In Bioremediation and Biotechnology: Sustainable Approaches to Pollution Degradation (pp. 1–26).
Carrard, N., Foster, T., & Willetts, J. (2019). Groundwater as a source of drinking water in Southeast Asia and the Pacific: A multi-country review of current reliance and resource concerns. Water, 11(8), 1605. https://doi.org/10.3390 /w11081605
Damalas, C. A., & Eleftherohorinos, I. G. (2011). Pesticide exposure, safety issues, and risk assessment indicators. International Journal of Environmental Research and Public Health, 8(5), 1402–1419.
Copyright
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.

