Biochemical characterization and genotypic identification in hybrid maize - Hema
Abstract
The study was conducted at V.C. Farm, MARS, Mandya during the rainy seasons of 2015 and 2016 to characterize and identify hybrid maize genotypes (variety: Hema) using biochemical approaches. Twenty-eight genotypes were evaluated through colour-based biochemical tests. In the NaOH test, 12 genotypes showed light yellow, 8 dark yellow, and the rest no reaction. The KOH test indicated 16 light yellow, 9 medium yellow, 1 dark yellow, and the remaining as absent. The standard phenol test classified 4 genotypes as brown, 14 light brown, 1 dark brown, and 9 as absent. The modified phenol test with 1% CuSO₄ showed 4 brown, 13 light brown, 2 dark brown, 8 light yellow, and 1 black genotype. Electrical conductivity was highest in MAI-143 and MAI-33174 (341.20 and 337.50 µS ppm⁻¹) and lowest in MAI-267 (188.59 µS ppm⁻¹). MAI-267 recorded the highest TDH activity (1.77), soluble protein (364.45 μg), and sugar content (21.95%), while MAI-143, MAI-204, MAI-194, and MAI-12 showed the lowest values. These results highlight significant biochemical variation among genotypes, supporting their use in hybrid identification and maize improvement programs.
Introduction
Maize (Zea mays L.) is one of the most important staple food crops globally, ranking third after wheat and rice. Its significance lies not only in its high production potential but also in its adaptability to a wide range of agro-climatic conditions. In India, maize plays a crucial role in the national economy, comparable to other major cereals such as rice, wheat, and millets. Apart from being a vital food source for humans, maize serves as a major component in animal feed and is widely used in industrial applications for producing starch, syrup, alcohol, acetic acid, lactic acid, and other derivatives. The quality of seed is a critical factor in ensuring successful crop establishment and productivity, particularly in maize. On-farm seed treatment techniques have been promoted among small-scale farmers to improve germination and early plant vigor, as treated seeds can absorb water more efficiently under field conditions, leading to faster and more uniform germination (Harris et al., 2007).
Traditional varietal characterization based on morphological traits, while useful, presents several limitations including environmental influence, seasonal dependency, space requirements, and time-consuming procedures. To overcome these challenges and accelerate the identification process, biochemical tests have emerged as efficient alternatives. These tests, including phenol reaction, modified phenol test, and alkali tests using NaOH and KOH, are rapid and effective for identifying genotypes based on colour changes in seeds and solutions upon chemical exposure. Such biochemical markers have proven valuable in detecting varietal mixtures and classifying a large number of genotypes into distinct groups, as demonstrated in crops like greengram (Chakrabarthy and Agrawal, 1990). Therefore, incorporating biochemical approaches offers a reliable and practical method for varietal characterization and purity assessment, especially in hybrid maize breeding programs.
Material and Methods
The study was conducted at the Zonal Agricultural Research Station (ZARS), V.C. Farm, Mandya, under the University of Agricultural Sciences (UAS), Bengaluru, during the rainy seasons of 2015 and 2016. A total of twenty-eight hybrid maize genotypes were obtained from the All India Coordinated Research Project (AICRP) on maize. The seeds were cleaned, dried to a safe moisture content of 10%, graded to uniform size, and used for characterization studies. Each genotype was sown in 4-meter-long rows with a spacing of 30 × 60 cm, and standard agronomic and plant protection practices were followed throughout the crop season. Biochemical characterization of the genotypes was performed using various standard tests, as detailed below:
Sodium Hydroxide (NaOH) Test
Seeds were soaked in 2% NaOH solution for two hours, and the resulting colour change in the solution was observed as per the method of Papp et al. (1997). Based on the intensity of colour, genotypes were categorized into three groups: light yellow, dark yellow, and dark red.
Potassium Hydroxide (KOH) test
Seeds were soaked in 4% KOH solution for two hours, and colour changes were recorded following the same reference method. Genotypes were grouped into four categories: light yellow, medium yellow, medium red, and dark red.
Standard phenol test
Seeds were pre-soaked in distilled water for 24 hours and then placed on filter paper moistened with 1% phenol solution. After 24 hours, seed coat colour changes were recorded and genotypes were classified into four groups: absent, light brown, brown, and dark brown.
Modified phenol test
Seeds were soaked in 0.5% CuSO₄ solution for 24 hours and then transferred to filter paper saturated with 1% phenol solution. After 24 hours, colour reactions were noted, and genotypes were categorized as light yellow, brown, dark brown, or black.
Electrical conductivity of seed leachate (µS ppm⁻¹)
The electrical conductivity (EC) of seed leachate was measured according to ISTA (2010). Twenty-five seeds per genotype, with three replications, were soaked in 50 ml of distilled water for 18 hours at 25 ± 1°C. After incubation, the leachate was decanted, and EC was measured using a digital conductivity meter (Model D1 9009) and expressed in µS ppm⁻¹.
Total Dehydrogenase (TDH) activity (A480 nm)
TDH activity was estimated as per Perl et al. (1978). Ten randomly selected seeds from each of three replications were pre-soaked in water for 24 hours. The seeds were then bisected longitudinally and soaked in 0.5% tetrazolium chloride solution, followed by incubation at 25 ± 1°C in the dark for six hours. After thorough washing, the red formazan pigment formed in the embryos was extracted with 5 ml of 2-methoxy ethanol over 6–8 hours in airtight containers. The extract’s absorbance was read at 480 nm using a spectrophotometer (Model Mini Spec 17), and TDH activity was expressed in absorbance units.
Results and Discussion
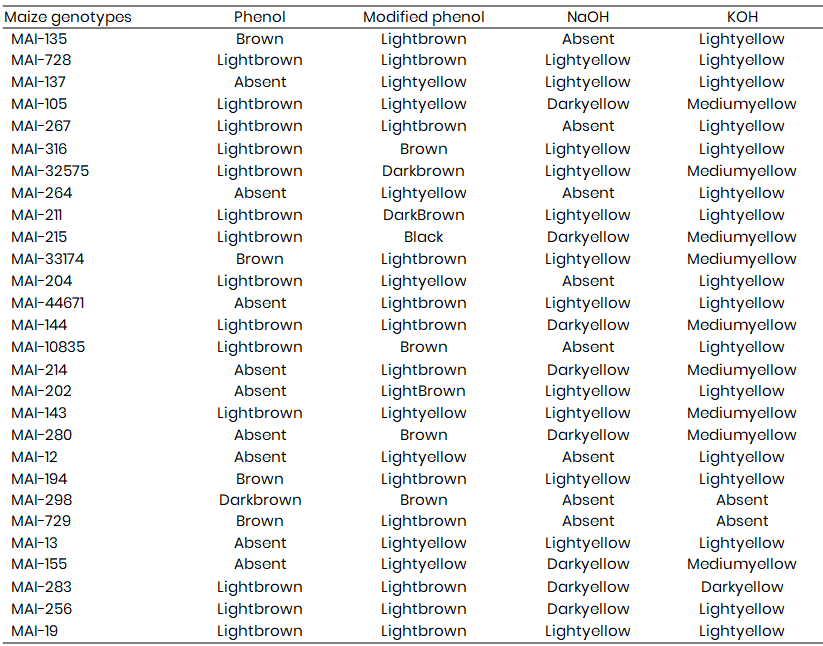
The biochemical characterization of twenty-eight maize genotypes was conducted using a series of rapid chemical tests to distinguish genotypic variability. The sodium hydroxide (NaOH) test categorized the genotypes into three distinct groups based on seed colour reaction. Among the genotypes, twelve (MAI-728, MAI-137, MAI-316, MAI-32575, MAI-33174, MAI-44671, MAI-202, MAI-143, MAI-211, MAI-194, MAI-13, and MAI-19) exhibited a light yellow reaction, while eight genotypes (MAI-280, MAI-105, MAI-215, MAI-144, MAI-214, MAI-155, MAI-283, and MAI-256) showed dark yellow (Table 1). The remaining genotypes were grouped under the absent category. The observed colour variation is attributed to the interaction of seed constituents, particularly secondary metabolites, with the alkaline medium, reflecting underlying genetic differences in enzyme systems (Chakrabarthy & Agrawal, 1990; Anithalakshmi, 2002; Thangavel et al., 2005).
In the potassium hydroxide (KOH) test, genotypes were grouped into four categories: light yellow, medium yellow, dark yellow, and absent. Sixteen genotypes, including MAI-135, MAI-728, MAI-137, MAI-267, MAI-211, MAI-204, MAI-44671, MAI-10835, MAI-202, MAI-12, MAI-194, MAI-13, MAI-256, MAI-19, MAI-316, and MAI-264, showed a light yellow reaction. Nine genotypes (MAI-280, MAI-105, MAI-32575, MAI-215, MAI-33174, MAI-144, MAI-214, MAI-143, and MAI-155) exhibited medium yellow, while MAI-283 alone showed dark yellow coloration. The rest were placed in the absent category. These reactions are indicative of seed chemical composition and enzymatic activity, which are genetically controlled, consistent with findings in wheat, sorghum, and rice (Dileepkumar et al., 2015).
The standard phenol test revealed phenol-based oxidative reactions on the seed coat, which were used to group genotypes. Four genotypes (MAI-135, MAI-33174, MAI-729, and MAI-194) showed a brown reaction; fourteen genotypes showed light brown (including MAI-728, MAI-105, MAI-267, MAI-316, MAI-32575, MAI-211, MAI-215, MAI-204, MAI-144, MAI-10835, MAI-143, MAI-283, MAI-256, and MAI-19), while MAI-298 alone showed a dark brown reaction. The remaining nine genotypes exhibited no response. The phenol reaction serves as a marker for polyphenol oxidase activity, an established trait used in varietal identification (Joshi & Banerjee, 1970), with similar observations reported in sorghum and maize (Thangavel et al., 2005).
In the modified phenol test with 1% CuSO₄, which enhances enzymatic reactions due to catalytic action of Cu²⁺ ions, the genotypes were grouped into five categories: brown, dark brown, light brown, light yellow, and black. Four genotypes (MAI-316, MAI-10835, MAI-280, and MAI-298) showed brown coloration; thirteen (including MAI-135, MAI-728, MAI-202, MAI-33174, MAI-44671, MAI-144, MAI-214, MAI-267, MAI-194, MAI-729, MAI-283, MAI-256, and MAI-19) were classified as light brown; two genotypes (MAI-32575 and MAI-211) were dark brown; eight (MAI-137, MAI-105, MAI-264, MAI-204, MAI-143, MAI-12, MAI-13, and MAI-155) exhibited a light yellow response, and MAI-215 alone showed a black reaction. These results align with earlier findings that Cu²⁺ enhances phenol oxidation and seed coat colour changes (Banerjee & Chandra, 1977; Dileepkumar et al., 2015).
Table 1: Response of maize genotypes to different rapid chemical tests
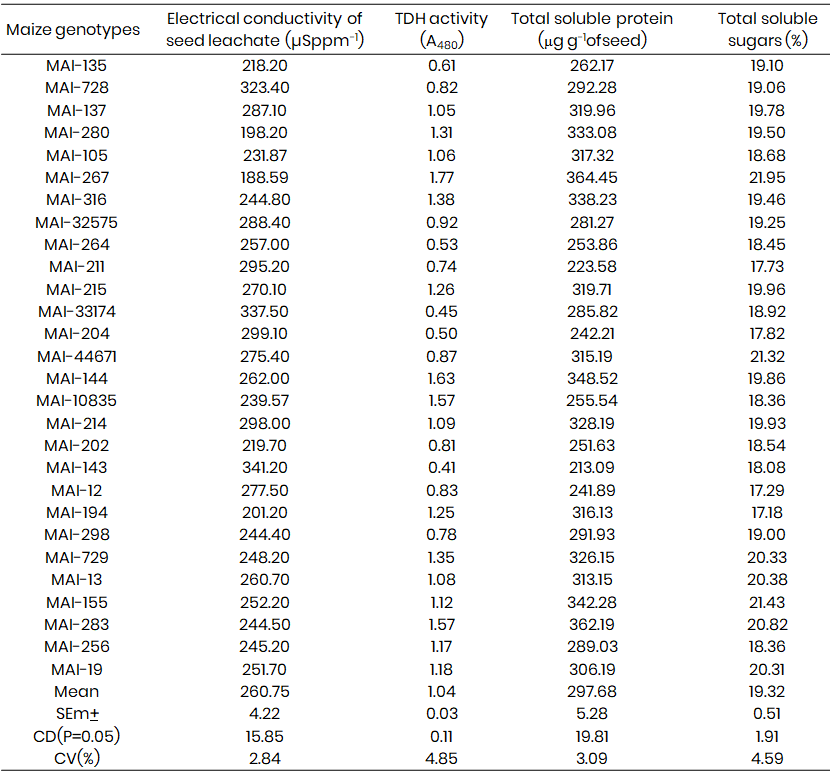
Significant differences were also observed in physiological parameters (Table 2). The electrical conductivity (EC) of seed leachate, an indicator of membrane integrity, was lowest in MAI-267 (188.59 µS ppm⁻¹), followed by MAI-280 (198.20 µS ppm⁻¹), indicating superior membrane stability. The highest EC was recorded in MAI-143 and MAI-33174 (341.20 and 337.50 µS ppm⁻¹), suggesting greater seed deterioration. These findings reflect differences in seed quality and viability.
The total dehydrogenase (TDH) activity, a measure of metabolic activity, also varied significantly. MAI-267 recorded the highest activity (1.77 A₄₈₀ nm), while the lowest was observed in MAI-143 and MAI-204 (0.41 and 0.50, respectively). This suggests a higher respiratory and metabolic efficiency in MAI-267, possibly due to a more robust enzyme system.
Regarding biochemical constituents, MAI-267 again recorded the highest soluble protein content (364.45 μg), while MAI-143 had the lowest (213.09 μg). In terms of soluble sugar content, MAI-267 was superior (21.95%), whereas MAI-194 and MAI-12 recorded the lowest levels (17.18% and 17.29%, respectively). These differences reflect genotypic variation in storage reserves and metabolic potential. Notably, genotypes such as MAI-267, MAI-155, MAI-283, MAI-144, MAI-729, MAI-280, and MAI-215 demonstrated both high protein and sugar content, making them promising candidates for future breeding programs aimed at improving seed quality traits.
Table 2: Variation in EC (µSppm-1), TDH activity (A480 nm), Total soluble protein (&m
References
Anithalakshmi, V. (2002). Characterization and identification of cultivars based on morphological and biochemical markers in rice (Oryza sativa L.) (Master's thesis, University of Agricultural Sciences, GKVK, Bangalore).
Banerjee, S. K., & Chandra, S. (1977). Modified phenol test for the varietal identification of wheat seed. Seed Science and Technology, 5(1), 53–60.
Chakrabarthy, S. K., & Agrawal, R. L. (1990). Identification of blackgram varieties III: Utilization of seedling growth response to added chemicals. Seed Research, 18(1), 34–39.
Dileepkumar, A. M., Vyakarnahal, B. S., Deshpande, V. K., Jagadeesha, R. C., Mukesh, L., Chavan, A. N., & Khajarubina, S. (2015). Characterization of traditional aromatic cultivars by chemical markers. International Journal of Advanced Research, 3(5), 103–107.
Harris, D., Rashid, A., Miraj, G., Arif, M., & Shah, H. (2007). On-farm seed priming with zinc sulphate solution: A cost-effective way to increase the maize yields of resource-poor farmers. Field Crops Research, 102, 119–127. https://doi.org/10.1016/j.fcr.2007.03.002
Joshi, M. G., & Banerjee, S. K. (1970). Genetics of phenol colour reaction in emmer wheats. Proceedings of the International Seed Testing Association, 35, 207.
Papp, E., Korosi, F., & Kemey, A. (1997). Identification of seeds of Brassica species on the basis of spectrophotometry of seed extracts. Seed Science and Technology, 25, 577–580.
Perl, M., Luria, I., & Gelmond, H. (1978). Biochemical changes in sorghum seeds affected by accelerated ageing. Journal of Experimental Botany, 29, 497–501. https://doi.org/10.1093/jxb/29.2.497
Thangavel, P., Bharathi, A., Natarajan, N., & Evera, T. (2005). Varietal grouping in sorghum by seed and seedling morphology and response to chemical testing. Karnataka Journal of Agricultural Sciences, 18(3), 664–672.
Copyright
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.

