Consequences of Niger (Guizotia abyssinica) crop on diversity of entomofauna
Abstract
Niger is primarily farmed during rainy season on about 0.52 million acres of land in India. Over 80% of the acreage and output is contributed by Madhya Pradesh, Maharashtra, and Orissa. Numerous beneficial traits, including as high biomass potential, tolerance to marginal soils, and pollinator appeal, make Niger Guizotia abyssinica an ideal dicot species to coexist alongside perennial warm-season grasses. Study illustrates the variety of visitors to flowers. The information was gathered through direct observation on two days a week for three months, from 8 am to 4 pm. There were 1162 individuals in all, representing 45 species, in five orders and 27 families. The family Apidae has greater number of individuals compared to any other family, followed by family formicidae and family lycaenidae. There has to be a greater environmental knowledge today regarding practical habitat management that could contribute to an increase in insect pollinators. In order to preserve pollinators and to fully utilize the potential of crop pollination, exploratory study should also be conducted.
Introduction
An oilseed crop known as Niger, or Guizotia abyssinica, is mostly grown as a marginal crop in India. It makes up 3% in India. It is grown in the Indian states of Andhra Pradesh, Madhya Pradesh, Orissa, Maharashtra, Bihar, Karnataka, Nagar Haveli, and West Bengal; Madhya Pradesh is the biggest of these. It is a dicotyledonous herb with a 2 m maximum height and moderate to good branching. The niger blossom's predominant colour is yellow with a touch of green. Ray florets in the heads range in size from 5 to 20 mm in length to 15 to 50 mm in diameter. Each of the two to three growing capitulate (heads) has a ray. The semi-spherical container has a diameter of 1-2 cm and a height of 0.5-0.8 cm. Involucral bracts are arranged in two rows on either side of the receptacle. The capitulum is composed of six to eight healthy female ray florets with narrowly elliptic, obovate ovules. The stigma is comprised of two 2 mm long coiled branches. The hermaphrodite disc florets, which normally number 40 to 60 per capitulum, are organised into three whorls. The disc florets have yellow to orange anthers, and the stigma is quite hairy.
Niger is typically grown in light, poor soils with a gritty texture. The Niger is mostly pollinated by insects, particularly bees, because it is a totally out-crossing plant with a self-incompatibility mechanism (Venkataramegowda et al., 2013). As a result of the mixing of one or more forage species, which may promote biodiversity and offer habitat for insects and thus improve the pollination services in agro ecosystems, ecosystem goods and services from biomass commodity production systems rose considerably (Patil and Jagdale, 2021) Insects that visit Niger flowers range widely, from Collembola to Hymenoptera (Kevan and Baker, 1984). Insects harvest nectar, pollen, floral tissues, shelter, partners, and oviposition locations from flowers.
Niger is a bioenergy crop that offers more environmental services, such as food and shelter for open-range species like birds and insects, and as a result might be a better option than huge biogas facilities because it is more advantageous to the environment. Plant and pollinator populations may drop in human-modified landscapes due to habitat loss and fragmentation, which may restrict pollination (Kremen, 2004). Threats to insect pollinators include loss of habitat, altered land use, excessive pesticide usage, and modern farming techniques.
The current study sought to examine the effects of the Niger on insect diversity because some plants can still support biodiversity. This work is significant because it has increased understanding of pollinators on the Niger in the Satara district, which will aid in the planning and execution of plant and pollinator conservation.
Materials and Methods
We investigated a seed-producing crop whose oil is generally extracted from the seeds. The investigation was conducted in the Karad, Khatav, and Patan tehsil of the Satara district's agricultural fields (Plate 2). Southern Maharashtra state of India. Karad Tehsil. The coordinates of Karad are 17.285°N 74.184°E. It measures 566 metres on average. The coordinates of Khatav are 17.6545°N 74.3614°E. It is 777 metres above sea level on average. The coordinates of Patan Tehsil are 17.37°N 73.9°E. It is 582 metres above sea level on average. Less rain fell on the agricultural fields, which had a 29°C temperature. The niger plant was grown in four distinct fields. There were roughly 2023.428 square metres of fields. From August through November 2020, prime flowering times, the investigation was carried out. For the pollinator survey, we set aside two 5 × 5 m plots at each location, one in each field.
Two times per week, during the three observation periods per day of 8 a.m. to 11 a.m. for the morning, 12 a.m. to 1 p.m., and 2 p.m. to 4 p.m. for the afternoon, pollinator visits to flowers were recorded. The following methods were used to count and gather data on the number of insects that visited the Niger flower reproductive whorls only.
Photos taken with a Canon EOS 200 camera were examined for evidence of flower visits.
1. Sweep net: Species were collected using a sweep net after being photographed and observed to determine how frequently they were visited. Sweeping insects were collected and put in plastic containers. The insects were collected, categorized, labeled, and preserved either dry-pinned or in 70% alcohol.
2. Collection by hand: Hand-collected insect pollinators were then placed in lethal bottles. The insects were prepared for pinning and kept in dry condition on a wooden box.
During their flowering season, the number of various insect pollinators of niger was investigated. Twenty flowers were chosen at random. A timer was used to time the number of different insects that visited all of the niger blooms in a square metre of space for two minutes each plant, every hour from 8 am to 4 pm. The information was saved for later analysis.
Identification
Collected insect pollinators were identified using standard manuals and keys found in Ananthkrishnan and David (2004).
Results and Discussion
In this study, 1162 individuals from 45 species (Plate 1a, 1b and 1c), representing five orders and 28 families, were found. These orders of insect visitors included the order Hymenoptera, (Apidae, Megachilidae, Halictidae, Formicidae, Thynnidae, Vespidae), Lepidoptera (Lycanidae, Pieridae, Nymphalidae, Crambidae, Choreutidae, Erebidae), Diptera (Muscidae, Syrphidae, Sphecidae, Tachinidae, Sarcophagidae, Rhiniidae, Limoniidae, Asilidae), Hemiptera (Pentatomidae, Coreidae,), Coleoptera (Scarabidae, Chrysomelidae, Phalacridae, Meloidae, Coccinellidae) (Table 1).
During the study, over different locations, all the insect pollinators observed were belonged to forty–five species, forty-one genera of twenty-eight families under five orders (Fig. 1). A maximum number of pollinator species belonged to the order Hymenoptera (sixteen species), followed by the order Diptera (ten species) followed by the order Lepidoptera (nine species) followed by Hemiptera (three species) and Coleoptera (seven species). Among the families, Apidae was found to be the abundant one comprising of seven species namely, Apis dorsata, Apis cerana, Apis florea, Apis andreniformis, Amegilla cingulata, Xylocopa latipes, Triepeolus eliseae. Followed by Lycaenidae comprising four species namely, Chilades pandava, Nacaduba kurava, Castalius rosimon, Spindasis vulcanus. The two families shared three species each i.e. Formicidae (Camponotus pennsylvanicus, Solenopsis invicta, Formica fusca) and Syrphidae (Episyrphus balteatus, Eristalinus arvorum, Chalcosyrphus femoratus). Four families shared two species each i.e. Megachilidae (Coelioxys elongata, Megachile inimica) Family Pentatomidae (Euthyrhynchus floridanus, Nezara viridula nymph), family Chrysomelidae (Bruchidius villosus, Acanthoscelides obtectus) and family Meloidae (Mylabris pustulata, Mylabris phalerata. Remaining families shared only one species each i.e. Halictidae comprising (Augochlora pura), Sphecidae (Chalybion californicum), Thynnidae (Myzinum quinquecinctum) Vespidae (Rhynchium oculatum), Asilidae (Mallophora leschenault), Muscidae (Musca domestica), Rhiniidae (Stomorhina lunata), Sarcophagidae (Sarcophaga bercaea), Tipulidae (Limonia phragmitidis), Tephritidae (Trupanea crassipes), Tachinidae (Carcelia iliaca), Nymphalidae (Hypolimnas bolina), Pieridae (Eurema hecabe), Crambidae (Spoladea recurvalis), Choreutidae (Saptha cypridia), Erebidae (Amata bicincta), Coreidae (Gonocerous acuteangulatus), Scarabidae (Gametis versicolor), Phalacridae (Phalacrus fimetarius) and Coccinellidae (Menochilus sexmaculata).
Painkra et al. (2015) found 15 species of insect pollinators / visitors on niger flowers namely, Apis florea and Apis dorsata, Danaus chrysippus, Eristalis sp., Musca domestica, Nazara virudula, Dysdercus cingulatus, Coccinella septumpunctata, Vespa cincta, Leptocorisa acuta, Amata passelis, Pelopidas mathias, Sarcophaga sp. and Chrysomya sp.
_page-0008090424100739-103-0.jpg)
Plate 1a: Insect pollinators of Black seed /Niger (Guizotia abyssinica).
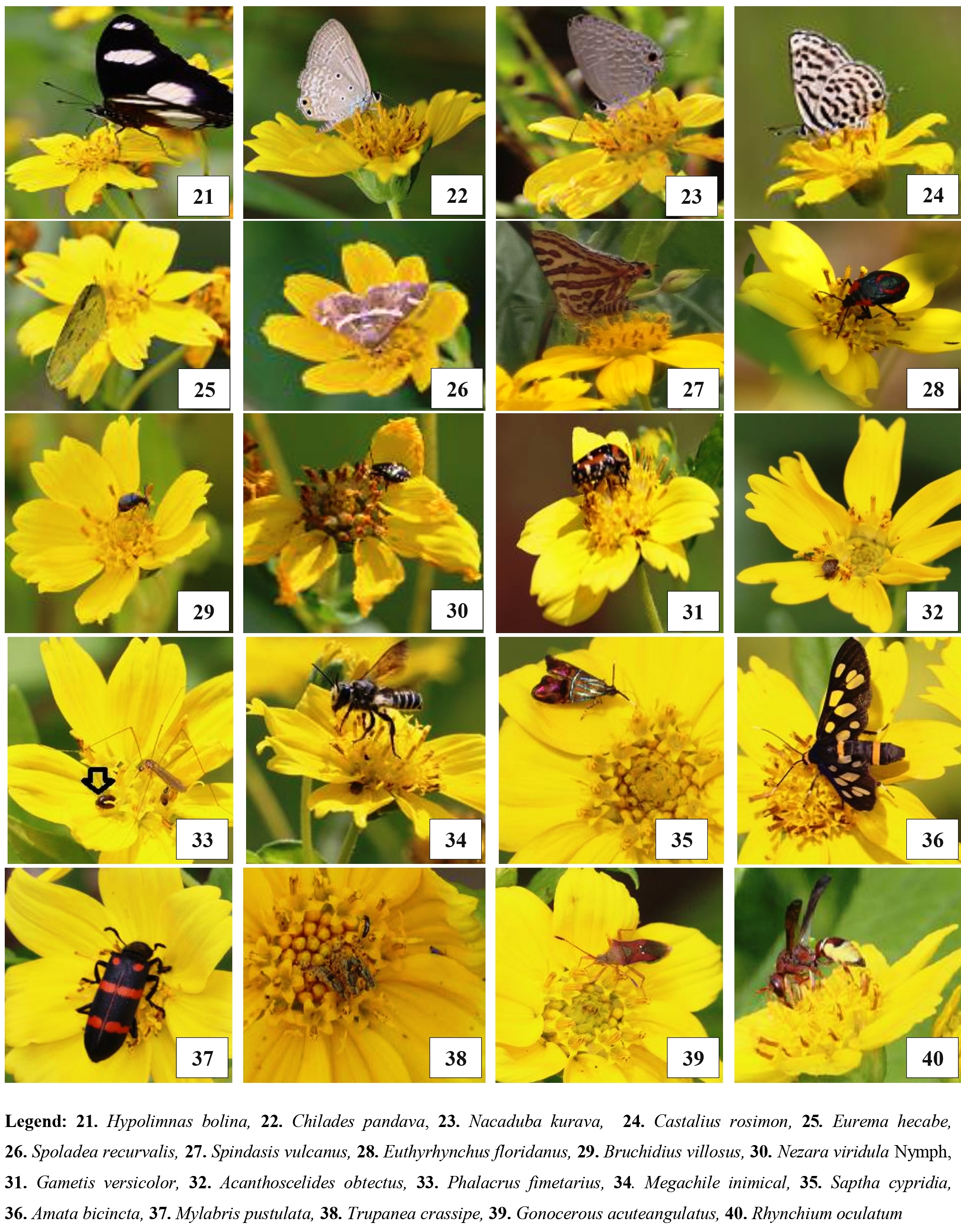
Plate 1b: Insect pollinators of Black seed /Niger (Guizotia abyssinica).

Plate 1c: Insect pollinators of Black seed /Niger (Guizotia abyssinica).
Thangjam et al. (2018) have recorded 19 species of insect pollinators visiting niger were belonged to ten families under five orders viz., Hymenoptera, Diptera, Coleoptera, Hemiptera and Lepidoptera. Andrena sp., A. florea, Apis dorsata, A. ceranaindica, Phytomia zonata, Eristalinus taeniops, E. punctulatus, Eristalinus arvorum, Erisyrphus balteatus, Micraspis discolor, Harmonia dimidiata, Popillia sp., Aulacophora foveicollis, Dysdercus cingulatus, Tajuria cippus, Chilades sp., Pieriscanidia indica, Nyctemera sp. and Amata sp.
The order Hymenoptera made up 78% of the species, followed by the orders Diptera (8%) and Coleoptera (7%), which were then followed by the orders Lepidoptera (5%) and Hemiptera (2%). (Fig. 2) Gebremedhn et al. (2014) observed that insect visitors from more than 11 species, representing 4 Orders and 7 Families, on G. abyssinica flowers. These observations supported the findings of Gebremedhn et al. (2014), who measured the abundance of insect pollinators in Ethiopia and discovered that the Order Hymenoptera had the highest abundance (81.6%), followed by Diptera (12%). Honeybees, in particular, accounted for 79% of all insect visitors. They also discovered that the time of day and flowering period had an impact on the abundance and diversity of insect pollinators. According to Kachhela and Pastagia (2018), A. dorsata was the most common flower visitor in Niger (9.33 bees/m2/5 minute), accounting for 60.74 percent of total flower visitors, followed by A. florea (2.30 bees/m2/5 minute) and A. cerana (1.17 bees/m2/5 minute), accounting for 14.97 and 7.62 percent of total flower visitors, respectively.
Thangjam et al. (2018) recorded that A. cerana was dominant forager (41.95%) followed by A. florea (20.13%) and A. dorsata (19.80%).
Table 1: Observations of number and nature of insect visitors on Niger plant.
Fig .1 Order and Family-wise insect visitors on
Tiwari et al. (2020) recorded Apis cerana indica (19.55), Apis dorsata (13.67), Apis mellifera (7.24), and Apis florea (1.81) as the most abundant pollinators. They also recorded Monarch butterfly – Danaus chrysippis (4.22), Rice skipper - Pelopidas mathias (4.88), Wasp - Vespa cincta (1.95), House fly - Musca domestica (3.28), Syrphid fly - Eristalis sp. (5.50), Blow fly- Chrysomya bezziana (1.82), Red cotton bug- Dysdercus cingulatus (1.97), and Tiger moth - Amata passelis (2.44) visiting on niger flower throughout the flowering period during rainy season.
The majority of insect visitors were found to visit flowers for pollen and nectar; very few insects visited flowers for warmth. Insects that visited flowers in search of prey included parasitoids and predators. For pollinators to complete their life cycle, food supplies, nesting materials, and nest sites must all be accessible (Potts, 2005; Winfree, 2010). The conservation of natural pollinators and the reproduction of planted species are better served by larger wildflower plantings with more varied flower species mixtures. Pollinator visitation rates fluctuated throughout the season, peaking around the time of flowering, according to Brett and Rufus (2014).
The richness of the associated invertebrate populations and for the birds depends greatly on the development of the ground vegetation within the plantations (Sage et al., 2006; Semere and Slater, 2007; Bellamy et al., 2009; Valentin et al., 2009). Many of these contain pest species that might harm the plantation, including leaf-eating beetles, Coleoptera: Chrysomelidae (Sage, 2008).
Fig. 2 Order wise Percentage of insect visitors
The availability of highly profitable mass flowering crops, such as oilseed rape, is directly correlated with the density of pollinator densities attracted by mass flowering (Westpal et al., 2003). Insects play a crucial role in many processes, including pollination (Ockinger and Smith, 2007; Ollerton et al., 2011), herbivory and detritivory (Yang and Gratton, 2014), and providing food for higher tropic levels of animals like amphibians, birds, and mammals. Their loss has detrimental effects on ecosystem functioning.
Because of modern agricultural methods, pollinators have insufficient access to the resources they need to survive, including food, nesting habitats, and other physical factors.
Plate 2: The agricultural fields where study was carried out.
Conclusion
Numerous creatures contribute to the ecological services that remove our garbage and put food on our tables. It is obvious that the majority of insects are essential for pollinating a variety of crop plants. It is beyond our wildest dreams how important pollinators are to maintaining biodiversity and pollinating an enormous array of flowering plants. Even though these services are essential to maintaining human existence, it can be challenging to give them a proper economic value, which can make conservation efforts more important.
There has to be a greater environmental knowledge today regarding practical habitat management that could contribute to an increase in insect pollinators. In order to preserve pollinators and to fully utilize the potential of crop pollination, exploratory study should also be conducted. Most notably, Niger (Guizotia abyssinica) plantations should be expanded since they have significant commercial and ecological importance as oil-producing plants that also play a significant role in maintaining insect biodiversity.
Acknowledgement
We are thankful to the farmers who had supported for this study; also we express our gratitude towards Mr. Hemant Patil and Mr. Sudhir Giri for their assistance during field work.
Conflict of Interest
None declared. The authors affirm no financial or personal relationships that could influence the objectivity or interpretation of the findings.
References
Ananthkrishnan T N and David B V. 2004. General and Applied Entomology, Second edition. New Delhi: Tata McGraw Hill publishing company limited.
Bellamy P E, Croxton P J, Heard M S, Hinsley S A, Hulmes L, Hulmes S, Nuttall P, Pywell R F and Rothery P. 2009. The impact of growing Miscanthus for biomass on farmland bird populations. Biomass and Bioenergy 33: 191-199.
Brett R B and Rufus I. 2014. Flower plantings increase wild bee abundance and the pollination services provided to a pollination-dependent crop. Journal of Applied Ecology 51: 890–898. Doi: https://doi.org/10.1111/1365-2664.12257
Gebremedhn H, Tadesse A and Belay T. 2014. Relating climatic factors to foraging behavior of honeybees (Apis mellifera) during blooming period of Guizotia abyssinica (LF). Livestock Research for Rural Development 26(4): 2-7.
Kachhela H R and Pastagia J J. 2018. Abundance of flower visitors and their foraging behaviour in Niger. Journal of Entomology and Zoology Studies 6(6): 562-564.
Kevan P G and Baker H G. 1984. Insects on flowers: pollination and floral visitations. In C. B. Huffaker and R. C. Rabb (eds.). Insect Ecology Journal 21: 607-631.
Kremen C, Williams N M, Bugg R, Fay J P and Robin W T. 2004. The area requirements of an ecosystem service: crop pollination by native bee communities in California. Ecology Letters 7: 1109-1119. Doi: https://doi.org/10.1111/j.1461-0248.2004.00662.x
Öckinger E and Smith H G. 2007. Semi-natural grasslands as population sources for pollinating insects in agricultural landscapes. Journal of Applied Ecology 44(1): 50–59.
Ollerton J, Winfree R and Tarrant S. 2011. How many flowering plants are pollinated by animals? Oikos 120(3): 321–326.
Painkra G P, Shrivastava S K, Shaw S S and Gupta R. 2015. Succession of various insect pollinators/visitors visiting on niger crop (Guizotia abyssinica cass.). International Journal of Plant Protection 8(1): 93-98.
Patil A H and Jagdale S P. 2021. Assessment of Habitat Availability and Diversity of Insect Pollinators on Mix and Isolated Allium cepa L. of Patan Tehsil in Satara District of Maharashtra (India). Research Journal of Agriculture Science 12(2): 548–551.
Potts S G, Vulliamy B, Roberts S, O'Toole C, Dafni A, Ne'eman G and Willmer P. 2005. Role of nesting resources in organising diverse bee communities in a Mediterranean landscape. Ecological Entomology 30: 78-85. Doi: https://doi.org/10.1111/j.0307-6946.2005.00662.x
Sage R B, Cunningham M and Boatman N. 2006. Birds in Willow short rotation coppice compared to other arable crops in central England and a review of bird census data from energy crops in the UK. Ibis 148: 184-197. Doi: http://dx.doi.org/10.1111/j.1474-919X.2006.00522.x
Sage R B. 2008. High invertebrates biodiversity in willow short rotation coppice can be protected when controlling chrysomelid pests by using a spatially targeted insecticide application. Proceedings Crop Protection in Northern Britain. pp. 33-38.
Semere T and Slater F M. 2007. Invertebrate populations in miscanthus (Miscanthus x giganteus) and reed canary-grass (Phalaris arundinacea) fields. Biomass and Bioenergy 31: 30-39.
Thangjam R, Ramya H R, Deka M K, Borah R K and Singh H R. 2018. Pollinator diversity and foraging behaviour of honey bee, Apis cerana indica on niger. Indian Journal of Entomology 80(3): 867-869.
Tiwari G K, Painkra G P, Bhagat P K, Painkra K L and Ameen G. 2020. Study of the insect pollinators visiting on Niger (Guizoti aabyssinica Cass.). Journal of Entomology and Zoology Studies 8(5): 2352-2357.
Valentin J, Duller C J and Metal H-J. 2009. The development of sustainable heat and power fueled by biomass from short rotation coppice in Wales. Aberystwyth University Report of the Helyg I Gymru /Willow for Wales 2004-2008 project. pp. 92.
Venkataramegowda, Sivaram, Jayaramappa, Koragandahalli, Menon, Anita, Ceballos and Ruben. 2013. Use of bee-attractants in increasing crop productivity in Niger (Guizotia abyssinica. L) Brazilian Archives of Biology and Technology 56: 365- 370.
Westpal C, Dewenter I S and Tscharntke T. 2003. Mass flowering crops enhance pollinator densities at a landscape scale. Ecology Letters 6: 961-965.
Winfree R. 2010. The conservation and restoration of wild bees. Annals of the New York Academy of Science 1195(1): 169-197. Doi: https://doi.org/10.1111/j.1749-6632.2010.05449.x
Yang L H and Gratton C. 2014. Insects as drivers of ecosystem processes. Current Opinion in Insect Science 2: 26–32. Doi: https://doi.org/10.1016/j.cois.2014.06.004
Copyright
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.

