Genetic analysis of Maize crop resistance to Polysora Rust: A comprehensive investigation
Abstract
This study investigates the inheritance pattern of Puccinia polysora rust resistance in maize, focusing on a segregating F2 population derived from a cross between the susceptible inbred line CM 202 and the resistant inbred line MAI 105. Disease severity was assessed using the Percent Leaf Area Covered (PLAC) method, revealing a spectrum of resistance within the population. Genetic analysis confirmed a single dominant gene controlling rust resistance, aligning with previous research indicating monogenic inheritance in maize. The identification of this dominant gene offers significant implications for breeding programs, enabling the development of rust-resistant varieties through marker-assisted selection and gene pyramiding. Additionally, the presence of moderate resistance and susceptibility suggests the involvement of modifiers or quantitative trait loci. Understanding these mechanisms is crucial for comprehensive rust resistance elucidation. Overall, this study provides valuable insights into the genetic basis of polysora rust resistance, informing breeding strategies to enhance maize productivity and resilience against rust diseases.
Introduction
Maize (Zea mays L.) is a fundamental crop globally, serving as a staple food for millions of people and playing a crucial role in various industries. Its versatility extends beyond dietary consumption, encompassing applications in livestock feed, biofuels, and industrial production. This cereal crop's adaptability to diverse climates and soil conditions underscores its significance in ensuring food security and economic stability worldwide. However, maize production faces formidable challenges, with diseases posing a significant threat to yields and food security. Among these pathogens, Polysora rust, caused by the fungus Puccinia polysora, emerges as a prominent adversary. This biotrophic fungus primarily targets maize leaves, manifesting as rust-like lesions that compromise photosynthetic efficiency and weaken the plant's overall vigor, ultimately leading to yield losses.
Understanding the genetic basis of resistance to Polysora rust is paramount for developing effective management strategies to mitigate its impact. Previous research endeavors have made notable progress in unraveling the genetic mechanisms underlying resistance, shedding light on the intricate interplay between host genetics and pathogen virulence. Studies have highlighted the existence of genetic diversity among maize genotypes concerning Polysora rust resistance. Chungu et al. (2007) conducted research on tropical maize inbred lines, revealing substantial variation in resistance levels. This diversity provides a valuable resource for breeding programs aimed at enhancing resistance in maize cultivars, thereby bolstering resilience against Polysora rust.
Furthermore, investigations into the resistance of maize hybrids and inbred lines have yielded insights into the genetic architecture of resistance traits. Munkvold and Mengistu (1998) evaluated various maize genotypes for their resistance to northern leaf blight and Polysora rust, identifying promising candidates with durable resistance. These findings underscore the importance of genotype-specific responses to pathogen challenges and emphasize the need for tailored breeding strategies to enhance resistance in maize cultivars. Characterization studies have contributed to elucidating the mechanisms underlying resistance to Polysora rust in maize. Takan et al. (1998) conducted comprehensive analyses to delineate the genetic basis of resistance traits, identifying key genomic regions associated with resistance. By dissecting the molecular pathways involved in host-pathogen interactions, such studies provide a foundation for targeted breeding efforts aimed at developing maize cultivars with enhanced resistance to Polysora rust.
Moreover, advances in molecular genetics and genomic technologies have facilitated the identification of candidate genes and markers linked to Polysora rust resistance in maize. These molecular tools offer valuable resources for marker-assisted selection (MAS) programs, enabling breeders to expedite the development of rust-resistant maize cultivars with improved agronomic traits.
In summary, maize stands as a cornerstone crop in global agriculture, but its productivity is threatened by diseases such as Polysora rust. Understanding the genetic basis of resistance to this pathogen is crucial for developing sustainable management strategies and ensuring food security. Through genetic diversity studies, characterization of resistance mechanisms, and leveraging molecular tools, researchers are making significant strides towards enhancing maize resilience to Polysora rust. Continued interdisciplinary efforts will be essential for harnessing the full potential of genetic resistance and safeguarding maize production against emerging challenges in the agricultural landscape.
Materials and Methods
The materials utilized, experimental procedures employed, and data analysis methods are comprehensively detailed below.
Selection of plant material
The base material for experimentation comprised one polysora rust-resistant inbred, MAI 105, and one susceptible inbred, CM 202. These lines were maintained through successive generations by selfing.
Crossing programme
The two selected inbred lines were planted during the late rainy season. The crossing procedure involved uniform silk cutting the previous evening, followed by crossing and pollination with pollen the following day between 8-10 AM. Subsequently, F1 progeny were harvested. The F1 generation was grown during the early rainy season and self-pollinated within the same season, with subsequent harvest. In late rainy season, the F2 population (consisting of 150 plants) was cultivated in three plots, each measuring 5 meters in length and arranged in four rows with a spacing of 70cm × 20cm. Polysora rust-susceptible genotype 219 J was planted as a border row, and artificial inoculation was conducted as previously described.
Assessment of Disease Severity
Disease severity/incidence data were recorded on individual plants at two intervals: first at anthesis and then at the dough stage, using a standard scale ranging from 1 to 5 (Cramer, 1967). Subsequently, these scores were converted to percentages of disease severity (0-100%) following the method outlined by James (1971).
Statistical Analysis
The segregation pattern in the cross was statistically analyzed using the Chi-square test to assess the inheritance pattern of the resistance trait.
By adhering to standardized protocols for plant material selection, crossing procedures, disease assessment, and statistical analysis, this study aimed to elucidate the inheritance pattern of polysora rust resistance in maize and contribute to the genetic improvement of maize cultivars for enhanced disease resistance.
Results and Discussion
The F2 population, comprising 150 plants resulting from the cross between the susceptible inbred line CM 202 and the resistant inbred line MAI 105, was meticulously cultivated and assessed for polysora rust disease severity on an individual plant basis. Disease severity was quantified using the PLAC (Percent Leaf Area Covered) method, which assigns a severity score ranging from 0 to 100% to each plant based on the proportion of leaf area affected by the disease.
To elucidate the inheritance pattern of polysora rust resistance, a genetic ratio analysis was conducted. The observed segregation ratio of 3 (resistant):1 (susceptible) was evaluated using the Chi-square test. The calculated Chi-square value was found to be lower than the tabulated Chi-square value, leading to the acceptance of the null hypothesis (Table 1). This statistical analysis supports the conclusion that the trait of polysora rust resistance in this population is governed by the control of a single dominant gene.
Table 1: Analysis of Polysora rust severity on F2 population
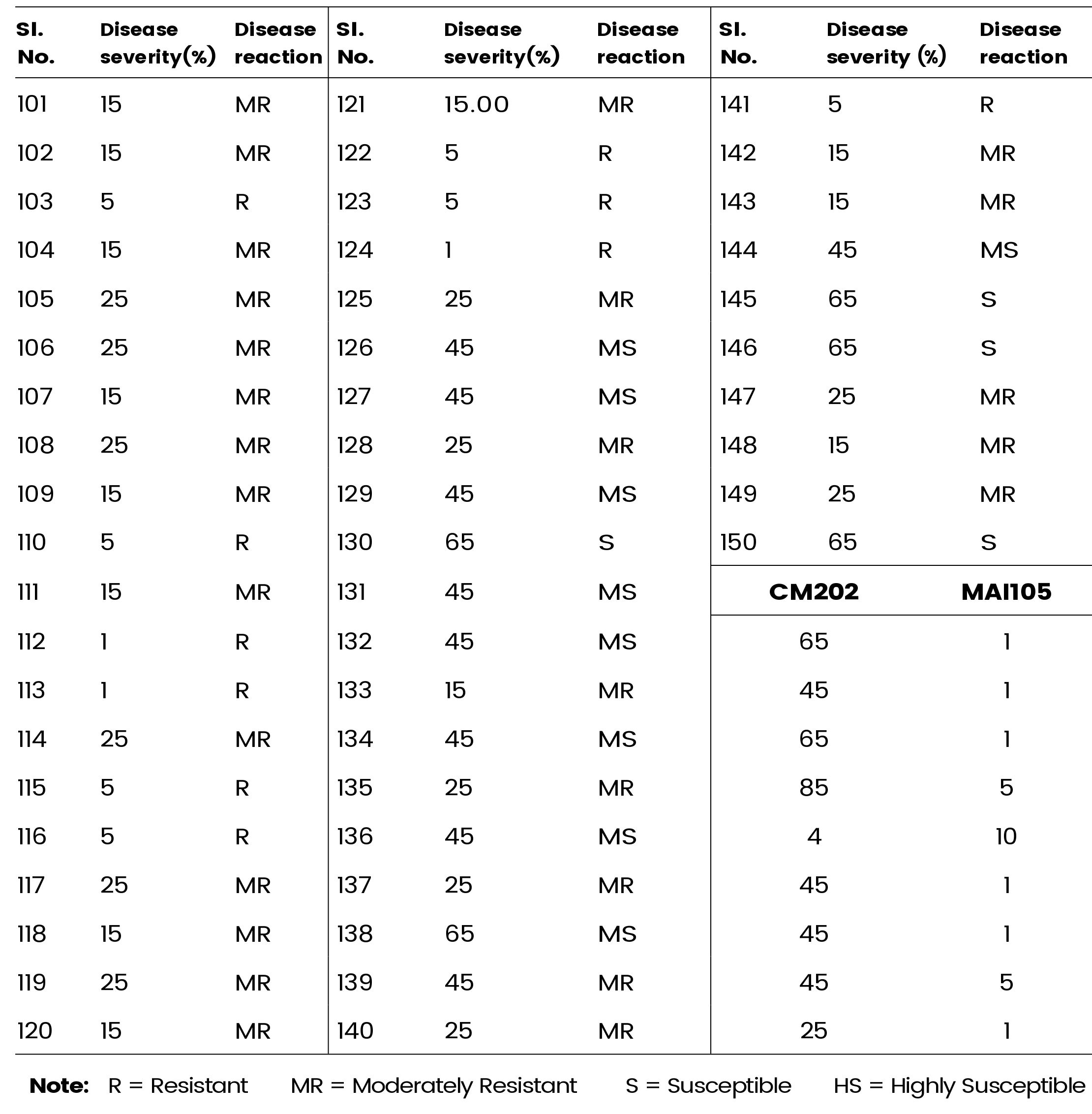
Table 2a and 2b presents the comprehensive data on disease severity within the F2 population. Among the 150 plants assessed, 53 demonstrated resistance to polysora rust, while 66 exhibited moderate resistance. Additionally, 27 plants displayed moderate susceptibility to the disease, while the remaining 5 plants were categorized as susceptible. This result underscores the genetic basis of polysora rust resistance in maize, providing valuable insights into the inheritance pattern of this trait.
Such findings hold significant implications for maize breeding programs aimed at developing cultivars with enhanced resistance to polysora rust, thereby contributing to sustainable maize production and food security.
Table 2a: Reaction of F2 population of maize line (CM-202×MAI-105) to Polysora rust
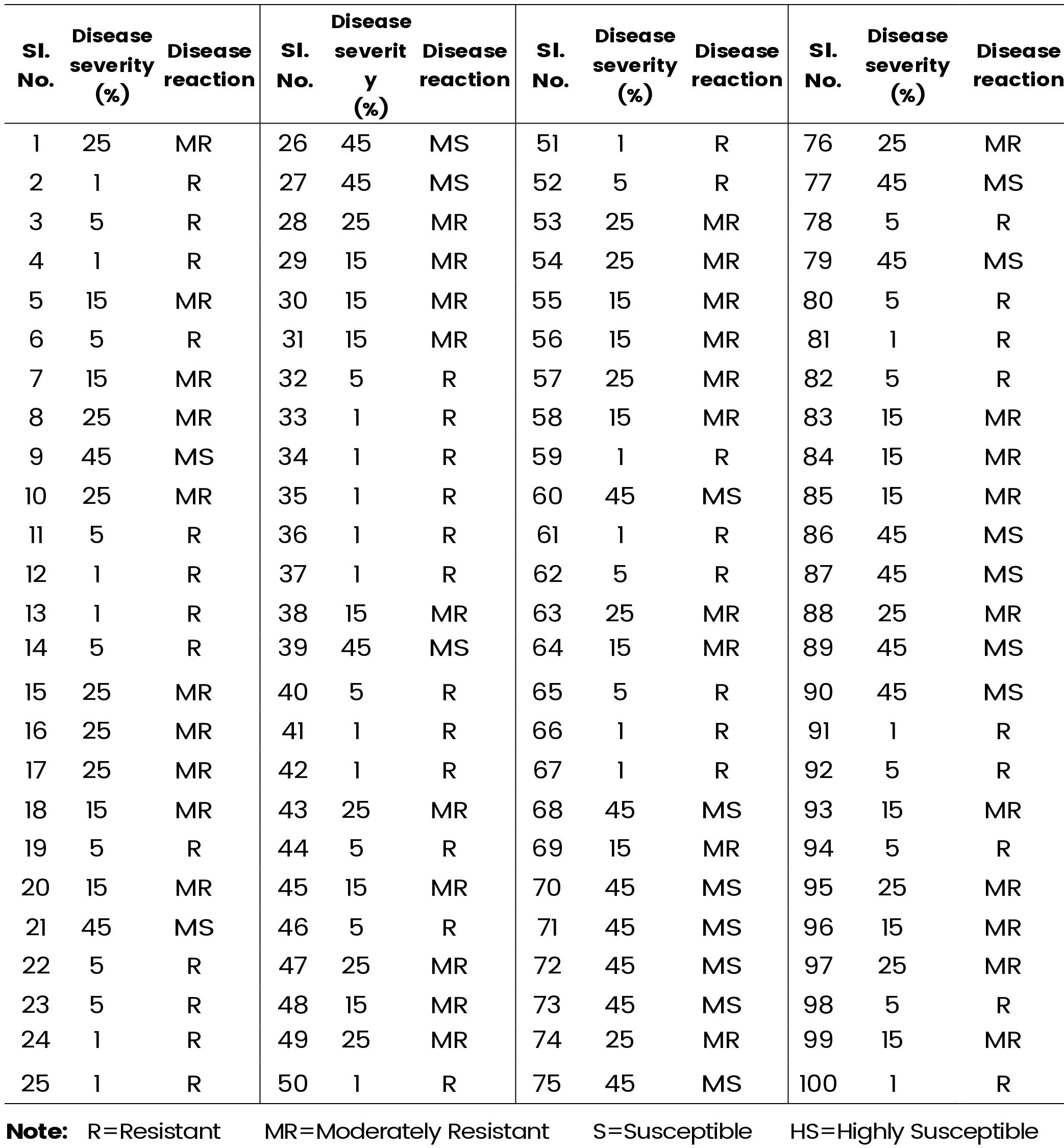
Table 2b: Reaction of F2 population of maize line (CM-202×MAI-105) to Polysora rust (Contd..)
Conclusion
The findings of this study shed light on the inheritance pattern of polysora rust resistance in maize, providing valuable insights into the genetic mechanisms underlying this important trait. The observed segregation ratio of 3 (resistant):1 (susceptible) in the F2 population suggests that the resistance to polysora rust is governed by the control of a single dominant gene. This finding aligns with previous research demonstrating the monogenic inheritance of rust resistance in maize (Takan et al., 1998; Chungu et al., 2007). Furthermore, understanding the genetic basis of rust resistance enables breeders to employ advanced breeding strategies such as gene pyramiding, which involves stacking multiple resistance genes to enhance durability and broaden the spectrum of resistance (Singh et al., 2017).
The moderate resistance observed in a substantial proportion of the F2 population suggests the presence of modifiers or quantitative trait loci (QTLs) influencing the expression of rust resistance. These modifiers may interact with the major resistance gene, influencing the degree of resistance conferred by the gene and contributing to the observed phenotypic variation (Kuchel et al., 2007). Further research is warranted to elucidate the genetic basis of moderate resistance and identify potential QTLs associated with this trait.
It is noteworthy that a small percentage of plants in the F2 population exhibited susceptibility to polysora rust despite originating from a resistant × susceptible cross. This phenomenon could be attributed to factors such as incomplete penetrance, environmental influences, or genetic background effects (Michelmore et al., 1991). Understanding the mechanisms underlying susceptibility in these individuals is essential for comprehensive elucidation of rust resistance in maize and may uncover novel genetic factors contributing to disease susceptibility.
The utilization of the PLAC method for disease severity assessment enabled precise quantification of rust symptoms, providing valuable data for genetic analysis. However, it is essential to acknowledge potential limitations associated with this method, such as subjectivity in symptom evaluation and variability in disease expression under different environmental conditions (Jeger et al., 2018). Integrating additional phenotypic and molecular approaches, such as histological analysis and gene expression profiling, could provide deeper insights into the mechanisms of rust resistance and complement the findings of this study.
In conclusion, the identification of a single dominant gene controlling polysora rust resistance in maize represents a significant advancement in our understanding of rust resistance genetics. This knowledge lays the foundation for targeted breeding efforts aimed at developing rust-resistant maize varieties with enhanced productivity and resilience. Continued research into the genetic basis of rust resistance, coupled with advancements in breeding technologies, holds promise for addressing the challenge of rust disease in maize and ensuring global food security.
References
Chungu C, Magorokosho E, Brown J A and Labuschagne M T. 2007. Genetic diversity in tropical maize inbred lines for resistance to Polysora rust (Puccinia polysora Underw.). Euphytica 158(1-2): 181-191.
Cramer H H. 1967. Plant protection and world crop production. Bayer, Leverkusen, West Germany.pp. 54.
James W C. 1971. An illustrated series of assessment keys for plant diseases, their preparation and usage. Canadian Plant Disease Survey 51: 39-65.
Jeger M J, Pautasso M, Holdenrieder O and Shaw M W. 2018. Modelling disease spread and control in networks: implications for plant sciences. New Phytologist 218(1): 41-53.
Kuchel H, Williams K J, Langridge P and Eagles H A. 2007. Genetic dissection of grain yield in bread wheat. II. QTL-by-environment interaction. Theoretical and Applied Genetics 115(7): 1015-1027.
Michelmore R W, Paran I and Kesseli R V. 1991. Identification of markers linked to disease-resistance genes by bulked segregant analysis: a rapid method to detect markers in specific genomic regions by using segregating populations. Proceedings of the National Academy of Sciences 88(21): 9828-9832.
Munkvold G P and Mengistu A. 1998. Resistance of maize hybrids and inbred lines to northern leaf blight and Polysora rust. Plant Disease 82(2): 179-184.
Singh V K, Khan A W, Jaganathan D, Thudi M, Roorkiwal M, Takagi H, Kumar V, Chitikineni A, Gaur P M, Sutton T, Terauchi R, Varshney R K. 2017. QTL‐seq for rapid identification of candidate genes for 100‐seed weight and root/total plant dry weight ratio under rainfed conditions in chickpea. Plant Biotechnology Journal 15(7): 907-919.
Takan J P, Lyon A K, Williams P H and Labuschagne M T. 1998. Characterization of resistance to Polysora rust in maize. Euphytica 99(3): 187-192.
Copyright
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.

