Abstract
Bacterial wilt caused by the soil-borne species complex Ralstonia solanacearum is a serious disease affecting potato (Solanum tuberosum L.) yield and quality in tropical, subtropical and warm temperate regions. In Madagascar, bacterial wilt occurs in all potato production areas causing severe crop losses and posing a serious threat to rural households’ food security. The present study carried out under rain-fed, field conditions at two sites located, in the Betafo district, in the Vakinankaratra region, the main potato production area aimed to assess biological control potential of two legume cover crops (Crotalaria spectabilis; Crotalaria grahamiana) as previous crop and intercrop against potato bacterial wilt. The experiment conducted on a moderately susceptible potato variety (Meva) on andosol was laid out in randomized complete block design with four treatments and four replicates. Treatments were consisted of potato sole cropping (control), potato mulching and intercropping with C. grahamiana, potato green manuring and intercropping with C. grahamiana and potato intercropping with Crotalaria spectabilis, respectively. Results showed positive effects of intercropping systems on potato yield parameters and biocontrol effects against potato bacterial wilt caused by strains of R. solanacearum phylotype I, biovar 3. Delayed bacterial wilt onset, decreased bacterial wilt incidence (-38 to -75%) and decreased infected tubers rates (-61 to -96%) were recorded in Crotalaria mixed cropping as well as increased potato total tubers (+107 to 145%) and marketable tubers (+125 to +170%) yields.
Introduction
Bacterial wilt caused by the soil-borne plant pathogenic species complex Ralstonia solanacearum is a serious disease of potato in tropical, subtropical and warm temperate regions (Hayward, 1991). The Ralstonia solanacearum species complex has broad plant host range comprising more than 200 species belonging to over 50 botanical families. They are responsible of several types of bacterial wilt disease on economic groups and ornamentals worldwide (Elphinstone, 2005).
Traditionally, the Ralstonia solanacearum species complex strains were classified into five (5) races based on their phenotypical properties, their differences in host range and into 5 biovars based on their differential ability to utilize carbon source from three hexose alcohols and three disaccharides (Hayward., 1994). Race 1 regroups strains that affect tobacco, potato, tomato, eggplant, diploid banana and many other Solanaceous crops and weeds. Race 2 strains affect triploid banana, plantain causing Moko disease as well as the ornamental plants, species of Heliconia spp. Race 3 strains mainly cause wilt on potato and tomato under temperate conditions. Race 4 strains and race 5 strains are specific to ginger and mulberry, respectively.
The Ralstonia solanacearum species complexe’s genetic diversity has been recently distributed into four phylogenetic groups (phylotypes) that correspond to their origin based on phylogenetic analysis of sequence data generated from 16S-23S ribosomal inter-genic spacer region as follows: Phylotype I, strains originating from Asia; Phylotype II, strains from the Americas; Phylotype III, strains from Africa and Phylotype IV, strains from Indonesia (Fegan and Prior, 2005). The phylotypes are subdivided into sequevar groups based on sequence variation in the hrpB and endoglucanase (egl) partial genes (Fegan and Prior, 2005).
The vascular pathogen penetrates host through roots natural openings such as emerging points of secondary roots and through nematode or insect-induced wounds (Vasse et al., 1995). Following penetration, the pathogen colonize and multiply massively in the xylem vessel, producing abundant exopolysaccharides before progressing into other parts of the pant. The production of exopolysaccharides combined with callose deposition from host, as a response to the infection, ultimately lead to xylem vessels obstruction and characteristic wilting symptoms development.
On potato, Ralstonia solanacearum strains cause wilting of aboveground vegetative parts and rotting of tubers at harvest and storage. Some strains can survive latently in potato tubers without triggering symptoms (Ciampi et al., 1981). The main source of inoculum are infected plant, contaminated soil, contaminated irrigation and surface water, latent infected potato seed tubers (Kelman et al., 1994).
In Madagascar, potato is an important food crops in the central Highlands, especially in the Vakinankaratra region, the main production region where area under potato production accounts more than 50 000 ha. In the Vakinankaratra region, potato is playing a key role in household food security as rice supplement or substitute during the lean period. In addition, potato is high value commercial crops generating supplementary incomes (CEFFEL, 2012).
Bacterial wilt is one of the most widespread, severe diseases affecting potato yield and quality and posing serious threat to rural households’ food security. Over the last decade, potato bacterial wilt outbreaks have been reported as more severe and more frequent.
Like other soil-borne disease, bacterial wilt is difficult to manage. No conventional method effectively controls it. Sub-groups of the species complex Ralstonia solanacearum encompasses strains that can survive for extended periods in soil and in roots and rhizosphere of many hosts (reservoirs) including weeds (Prior et al., 1990). Recent trends in bacterial wilt control are mainly focused on integrative measures including sustainable, ecological-friendly cultural practices based on sanitizing crops. These methods mostly rely upon enhancing host plant’s resistance and promoting soil health through decreasing infectious potential of soil and modifying soil microbial communities for disease suppression.
The present field study aimed at assessing biological control potential of two legume (Papilionaceae), sanitizing crops Crotalaria grahamiana, Crotalaria spectabilis, against potato bacterial wilt. Crotalaria grahamiana, a perennial species, is generally opportunistic, widespread throughout the Highlands and Crotalaria spectabilis, an annual species that is geographically restricted to the Midwestern part of the country. Both Crotalaria species are widely used in farming systems as green manure crop, cover crop and hedgerow crop.
Materials and Methods
Experimental sites
The present field study was carried out at two sites located at the rural Commune of Mandritsara (19°49'39'' S; 46°53'19'' E, 1,576 masl) and the rural Commune of Alakamisyanativato (19°53'01'' S; 46°54'20'' E, 1524 masl), in the Betafo District, in the Vakinankaratra region, in the Highlands of Madagascar, over one rainy season, from November 2020 to May 2021.
The climate is altitude tropical with a dry and cool season extending from May to September with minimal daily temperature ranging from 6°C to 9°C and a rainy and warm season extending from October to April with average daily temperature ranging from 13.2°C to 26°C. Mean annual rainfall averages exceed 1300 mm (Ahmim-Richard et al., 2018).
Soil type at the two sites are andosol, acidic or moderately acidic, relatively rich in carbon and in nitrogen, with a satisfactory C/N ratio, moderately rich in phosphorus and in potassium, with a loamy sand texture (Table 1).
Treatments and experimental design
The perennial (Crotalaria grahamiana) and the annual (Crotalaria spectabilis) herbaceous legume cover crops and the Meva potato cultivar, moderately susceptible to bacterial wilt caused by Ralstonia solanacearum were used for the field experiment. This potato cultivar is particularly appreciated by potato growers owing to its agronomic traits (earliness, 150 days of growth, fair storability), its technological characteristics (higher dry matter content, suitability to frying) and its higher economic value on domestic markets.
The experimental design was a randomized complete block design with four treatments and four replicates. Treatments were consisted of T1: potato sole cropping, T2: potato mulching and intercropping with C. grahamiana, T3: potato green manuring and intercropping with C. grahamiana, T4: potato intercropping with C. spectabilis. Each of the elementary plots contained 40 plants of potato planted at 0.7 × 0.3 m rows, under standard fertilizer composed of 10 t ha-1 of farmyard (cattle) manure and 33 kg N + 66 kg P2O5 + 48 kg K2O ha-1 in the form of NPK complex 11-22-16 applied at potato planting time complemented with additional 46 kg N ha-1 in the form of urea applied four weeks after potato planting during earthing up time (15-20 cm).
Under intercropping system, Crotalaria sp. seeds were sown in alternate rows (0.7×0.3m), in drill sowing pattern, 12 weeks prior to potato planting (previous cropping). Six weeks after sowing, C. grahamiana aerial biomass was entirely cut down and either allowed to die back to form a mulch (T2) or incorporated into the soil (T3) four weeks before potato planting while the root systems remain in the soil.
Sampling, measurement and analysis
Potato yield parameters measurement
At harvest (twelve weeks after planting), potato yield parameters [total number and yield of tubers per plant; total number and yield of commercial tubers (≥ 28 mm diameter) per plant] were measured on 12 potato plants located in the central two rows.
Bacterial wilt incidence monitoring
In each plot, bacterial wilt incidence was monitored at 5, 6, 7 and 8 weeks after potato planting using the formula:
Incidence (100%) = Σ wilted plants × 100 / total plants
At harvest, infected potato tubers percentages for each plot were calculated.
Isolation and characterization of presumptive Ralstonia solanacearum strains
Bacterial strains were isolated from stem, tuber vascular tissue of diseased potato plants.
Serial dilutions from stem, tuber vascular tissue and soil samples were spread onto semi-selective medium (SMSA: modified Sequiera Medium South Africa) (Elphinstone et al., 1996) and onto the triphenyltetrazolium chloride (TZC) medium.
Presumptive colonies of R. solanacearum developing the typical white with pink centres, irregularly-shaped, fluidal morphotype were subcultured and purified on TZC medium. Pure bacterial isolates were, subjected to biochemical characterization (biovar determination) and species and Phylotype-levels molecular characterization.
Biochemical characterization or biovar determination aimed at testing the ability of presumptive R. solanacearum strains to produce acids on Ayers basal media amended with three hexose alcohols (mannitol, sorbitol, dulcitol) and three disaccharides (cellobiose, lactose and maltose) as carbon source (French et al., 1993). Molecular characterization was carried out at species and phylotype level using reference strains.
DNA extraction from presumptive isolates of R. solanacearum
A loopful (1µl) of presumptive RSSC single colonies from a CPG plate was suspended in 100µl molecular biology grade water and boiled at 95°C for 15 min on a thermal cycler for DNA extraction (Weller et al., 2000).
PCR detection of RSSC and phylotype identification
DNAs from the boiled presumptive Ralstonia isolates are first screened using RSSC-specific primer pairs 759/760 of Opina et al. (1997). Phylotypes are screened by multiplex PCR combining the four phylotype-specific primers: N: mult21:1F (phylotype I), N: mult21:2F (phylotype II), N:mult23:AF (phylotype III), N:mult22:InF (phylotype IV) and the species-specific reverse primer Nmult22:RR as described by Fegan and Prior (2005).
The PCR is run with the following cycling conditions: initial denaturation of 15 min at 95°C; 30 cycles of 30 s at 94°C, 1 min at 59°C and 1 min at 72°C and final extension of 10 min at 72°C. The PCR reaction reagents, volumes and protocol were as described by Abdurahman et al. (2019). PCR products were visualized through electrophoresis, after application of an electric current of 90 volts for 40 minutes.
Statistical analysis
Para-metric data were subjected to analysis of variance followed by means separation according to the Tukey’s honestly significant difference test (p<0.05). For non-parametric data, Kruskal-Wallis test (α = 0.05) and then a pairwise comparison test was performed (α = 0.05) using the software XLSTAT. Data in percentages were transformed into logarithmic (Log 10) data prior to analysis of variance.
Results and Discussion
Biovar determination, species confirmation and phylotype identification
Biochemical tests revealed that all presumptive strains of Ralstonia solanacearum isolated from stem, tuber vascular tissue of symptomatic potato plants were capable of using all disaccharides and all hexose alcohols except inositol as carbon source.
Therefore, they can be classified into biovar 3. Molecular characterization carried out at species and phylotype level revealed 280bp fragment and 144bp fragment, respectively (Fig. 1 and 2). Ralstonia solanacearum strains belong to Phylotype I.
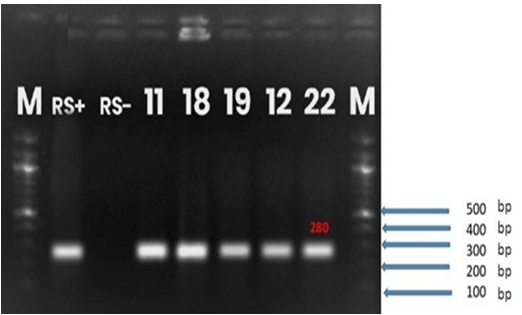
Fig.1: Gel electrophoresis of PCR amplification products
Lane M: DNA Marker; lane RS+: reference strain of R. solanacearum ; lane RS- :negative control, lane 18 : positive sample of R. solanacearum isolated from Alakamisyanativato, Betafo; 22: positive sample isolated from Mandritsara, Betafo
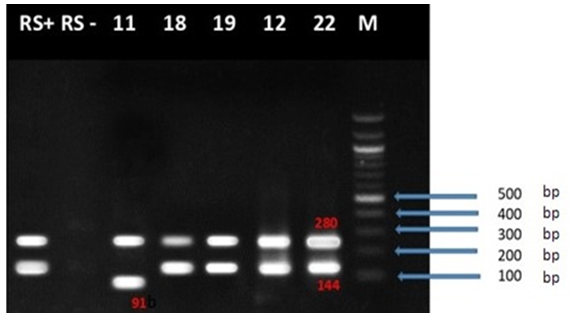
Fig. 2: Gel electrophoresis of Multiplex PCR amplification products
Lane M: DNA Marker; lane RS+: reference strain of R. solanacearum ; lane RS- :negative control, lane 18 : positive sample of R. solanacearum isolated from Alakamisyanativato, Betafo; 22: positive sample isolated from Mandritsara, Betafo
Potato agronomic parameters
At harvest, twelve (12) weeks after planting, potato total tubers and marketable yields per plant were significantly different between sites, treatments and their interactions. Over the two sites, intercropping systems gave +107 to +145% total potato tubers yield advantage over potato sole cropping. The highest yield advantage was recorded for soil treatment under mulching and intercropping with Crotalaria grahamiana (Fig. 3).
With regards to potato marketable tubers yield per plant, they are significantly (p<0.05) different between sites, treatments and their interactions. Over the two sites, maximum potato marketable tubers yield advantage was recorded for soil treatment under mulching and intercropping with Crotalaria grahamiana. Average potato marketable tubers fresh weight was +125 to +170% superior under intercropping systems (Fig. 3)
Potato bacterial wilt incidence
Bacterial wilt incidence was recorded from 5 to 8 weeks (37 to 56 days) after potato planting.
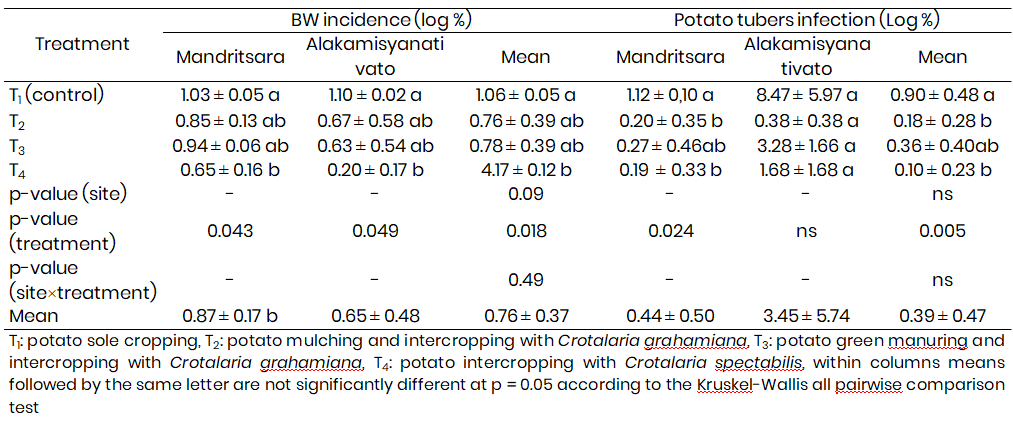
At both sites, bacterial wilt incidence increased over the time. Under intercropping system with Crotalaria spectabilis, bacterial wilt symptoms onset was delayed about one week at the Alakamisyanativato site where the legume cover crops grew rapidly. At 8 weeks after potato planting, cumulative bacteria wilt incidence varied significantly (p<0.05) between sites, treatments and their interaction. Over the two sites, bacterial wilt incidence was significantly declined by -38 to -75%, the lowest incidence was recorded for intercropping system with Crotalaria spectabilis (Table 2).
At harvest, bacterial wilt infected potato tubers percentage was decreased by -84 to -91% and -61 to -96% under soil treatment with mulching and intercropping of Crotalaria grahamiana and soil treatment with intercropping of Crotalaria spectabilis as compared to potato sole cropping, at the Mandritsara site and the Alakamisyanativato site, respectively. Over the two sites, averaged potato bacterial wilt incidence was -76 to -93% lower (Table 2).
Intercropping of moderately susceptible cultivar Meva of potato either with annual legume cover crops Crotalaria spectabilis or perennial legume cover crops Crotalaria grahamiana, on andosol showed potential to promote potato growth and control of bacterial wilt caused by strains belonging to phylotype I and biovar 3 of the species complexe Ralstonia solanacearum. Potato yield parameters in terms of total and marketable potato tubers yields were positively affected in significant way by intercropping.
Potato growth stimulation can be associated with positive effects of intercropping on soil physico-chemical properties leading to improved uptake of nutrients.
Positive effects of potato intercropping with legume cover crops were reported by other researchers (Guitari et al., 2018). Field experiment carried out in Kenya on rainfed potato intercropped with lima bean and dolichos revealed benefits of intercropping such as enhanced radiation interception and use efficiency, soil temperature, water content and productivity optimization. However, results obtained from the present experiment revealed that intercropping of potato with Crotalaria spp., particularly with Crotalaria spectabilis has great potential to control potato bacterial wilt through delayed disease onset, reduced incidence of bacterial wilt on potato aerial plant part (-75%) and tubers (-96%) (Nyawade et al., 2019).
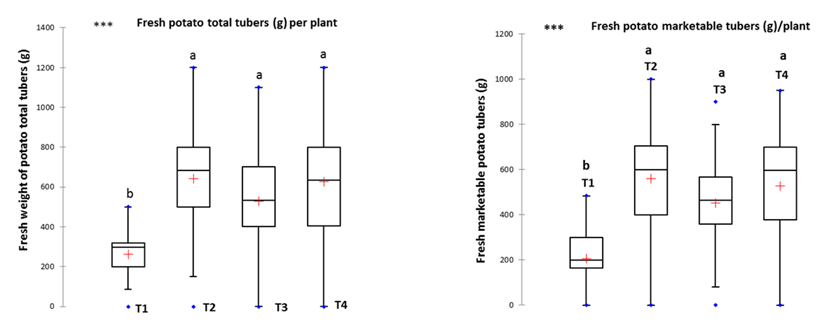
Fig. 3: Potato total tubers yield (left) and marketable tubers yield (right)
(T1: potato sole cropping, T2: potato mulching and intercropping with Crotalaria grahamiana, T3: potato green manuring and intercropping with Crotalaria grahamiana, T4: potato intercropping with Crotalaria spectabilis, within columns means followed by the same letter are not significantly different at p = 0.05 according to the Kruskel-Wallis all pairwise comparison test)
Table 2: Cumulative potato bacterial wilt (BW) incidence and infected tubers
Similar results were observed from a field experiment carried out in bacterial wilt endemically-infested field in Uganda, aiming at testing the effect of Crotalaria falcata as fallow and potato intercrop. Significant reduction of potato bacterial wilt incidence by 85% was recorded under soil fallowing with Crotalaria falcata and reduction of infected potato tubers by more than 94%. Reduced bacterial wilt incidence was coupled with suppression of latent R solanacearum infection after 6 to 12 months potato intercropping with C. falcate (Kakuhenzire et al., 2013).
In addition, results from a field experiment carried out in Martinique reported potato bacterial wilt onset delay of 25 days and reduction of bacterial wilt incidence up to 70% through previous cropping of C. spectabilis and C. juncea (Deberdt et al., 2015).
Besides having soil physico-chemical properties improving effects, Crotalaria spp. have suppressive effects on plant parasitic nematodes (Wang et al., 2002). They are generally poor host or non-host to many plant parasitic nematodes producing secondary metabolites, allelochemicals (pyrrolizidine) toxic to nematodes and bacteria (Joosten and Van Veen, 2011). Moreover, Crotalaria species are able to stimulate nematode-antagonistic microorganisms, hence preventing bacterial wilt infection through nematode-induced wounds. Other studies reported that soil organic matter increase was correlated with growth of facultative bacteria which compete with R. solanacearum strains (Cardoso et al., 2006). However, Vanitha et al. (2012) revealed that Crotalaria grahamiana exhibit phenolic compounds (flavonoids) in its different parts with significant antimicrobial activity.
Biological control effects of Crotalaria spectabilis and Crotalaria grahamiana against bacterial wilt can be associated with various mechanism such as potato nutritional quality and disease resistance enhancement through improved soil chemical and physical properties, or soil disease suppression through either diversity and composition of soil microbial communities modification or R. solanacearum specific antagonistic strains stimulation (Cardoso et al., 2006). Bacterial wilt symptoms apparition and intensity are dependent on several factors including host characteristics (species, cultivar and physiological stage), R. solanacearum strain type, level of inoculum and interactions with the environment (temperature, humidity, type of soil) (Van et al., 2004).
Acknowledgements
The authors appreciate support through funding from International Research Centre IRDC-Canada, managed by the Organization for Women in Science for the Developing World.
References
Abdurahman, A., Parker, M. L., Kreuz, J., Elphinstone, G., Struik, P. C., Kigundu, A., Arengo, E., & Sharma, K. (2019). Molecular epidemiology of Ralstonia solanacearum species complex strains causing bacterial wilt of potato in Uganda. Phytopathology, 109(11), 1922–1931.
Ahmim-Richard, A., Bodoy, A., & Penot, E. (2018). Caractérisation et typologie des exploitations agricoles dans le Vakinankaratra et l’Amoron’i Mania, Madagascar. Document de travail BV Lac. Phytopathology, 25.
Cardoso, S. C., Soares, A. C. F., Brito, A. D. S., Laranjeira, F. F., Ledo, C. A. S., & dos Santos, A. P. (2006). Control of tomato bacterial wilt through the incorporation of aerial part of pigeon pea and crotalaria to soil. Summa Phytopathologica, 32, 27–33.
CEFFEL. (2012). Étude de la filière légumes sur les Hautes Terres de Madagascar – régions Analamanga, Itasy, Vakinankaratra, Amoron’i Mania (pomme de terre, tomate, oignon, carotte, haricot vert et chou).
Ciampi, L., Sequeira, L., & French, E. R. (1981). Latent infection of potato tubers by Pseudomonas solanacearum. American Potato Journal, 57, 377–386.
Deberdt, P., Goze, E., Coranson-Beaudu, R., Perrin, B., Fernandes, P., Lucas, P., & Ratnadass, A. (2015). Crotalaria spectabilis and Raphanus sativus as previous crops show promise for the control of bacterial wilt of tomato without reducing bacterial populations. Journal of Phytopathology, 163(5), 377–385.
Elphinstone, J. G. (2005). The current bacterial wilt situation: A global overview. In C. Allen, P. Prior, & A. C. Hayward (Eds.), Bacterial wilt disease and the Ralstonia solanacearum species complex (pp. 9–28). APS Press.
Elphinstone, J. G., Hennessy, J., Wilson, J. K., & Stead, D. E. (1996). Sensivity of different methods for the detection of Ralstonia solanacearum in potato tuber extracts. EPPO Bulletin, 26(3–4), 663–678. https://doi.org/10.1111/j.1365-2338.1996.tb00968.x
Fegan, M., & Prior, P. (2005). How complex is the Ralstonia solanacearum species complex? In C. Allen, P. Prior, & A. Hayward (Eds.), Bacterial wilt disease and the Ralstonia solanacearum species complex (pp. 449–461). APS Press.
French, E. R., Aley, P., Torres, E., & Nydegger, U. (1993). Diversity of Pseudomonas solanacearum in Peru and Brazil. ACIAR Proceedings, 45, 70–77.
Guitari, H., Karanja, N. N., Gachene, C. K. K., Kamau, S., Sharma, K., & Schulte-Geldermann, E. (2018). Nitrogen and phosphorus uptake by potato (Solanum tuberosum L.) and their use efficiency under potato-legume intercropping systems. Field Crops Research, 222, 78–84.
Hayward, A. (1994). The hosts of Pseudomonas solanacearum. In A. C. Hayward & G. L. Hartman (Eds.), Bacterial wilt: The disease and its causative agent, Pseudomonas solanacearum (pp. 9–24). CAB International.
Hayward, A. C. (1991). Biology and epidemiology of bacterial wilt caused by Pseudomonas solanacearum. Annual Review of Phytopathology, 29, 67–87.
Joosten, L., & Van Veen, J. (2011). Defensive properties of pyrrolizidine alkaloids against microorganisms. Phytochemistry Reviews, 10, 127–136.
Kakuhenzire, R., Lemaga, B., Kashaija, I., Ortiz, O., & Mateeka, B. (2013). Effect of Crotalaria falcata in crop rotation and fallowing on potato bacterial wilt incidence, disease severity, and latent infection in tubers and field soil. Biopesticides, 9(2), 182–194.
Kelman, A., Hayward, A. C., & Elphinstone, J. G. (1994). Ralstonia (Pseudomonas) solanacearum. In G. L. Hartman, J. B. Sinclair, & J. C. Rupe (Eds.), Compendium of soybean diseases (pp. 35–37). APS Press.
Nyawade, S. O., Karanja, N. N., Gachene, C. K. K., Gitari, H., Schulte-Geldermann, E., & Parker, M. L. (2019). Intercropping optimizes soil temperature and increases crop water productivity and radiation use efficiency of rainfed potato. American Journal of Potato Research, 96, 457–471.
Opina, N., Tavner, F., Hollway, G., Wang, J. F., Li, T. H., Maghirang, R., et al. (1997). A novel method for development of species and strain-specific DNA probes and PCR primers for identifying Burkholderia solanacearum. Asia Pacific Journal of Molecular Biology and Biotechnology, 5, 19–30.
Prior, P., Steva, H., & Cadet, P. (1990). Aggressiveness of strains of Pseudomonas solanacearum from the French West Indies (Martinique and Guadeloupe) on tomato. Plant Disease, 74, 962–965.
Vanitha, S. M., Niranjana, S. R., Umesha, S., & Anandaraj, M. (2012). Isolation of flavonoids and biological activities of Crotalaria grahamiana. Journal of Chemical and Pharmaceutical Research, 4(7), 3665–3671.
Van Overbeek, L. S., Bergervoet, J. H. H., Jacobs, F. H. H., & van Elsas, J. D. (2004). The low-temperature-induced viable-but-nonculturable state affects the virulence of Ralstonia solanacearum biovar 2. Phytopathology, 94, 463–469.
Vasse, J., Frey, P., & Trigalet, A. (1995). Microscopic studies of intercellular infection and protoxylem invasion of tomato roots by Pseudomonas solanacearum. Molecular Plant-Microbe Interactions, 8, 241–251.
Wang, K., Sipes, B. S., & Schmitt, D. P. (2002). Crotalaria as a cover crop for nematode management: A review. Nematropica, 32(1).
Weller, S. A., Elphinstone, J. G., Smith, N. C., Boonham, N., & Stead, D. E. (2000). Detection of Ralstonia solanacearum strains with a quantitative, multiplex, real-time fluorogenic PCR (TaqMan) assay. Applied and Environmental Microbiology, 66, 2853–2858.
Copyright
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.

