Evaluation of phosphorus solubilising bacterial strain on growth and yield of Maize (Zea mays L.)
Abstract
A field experiment was conducted to evaluate the effectiveness of a phosphate-solubilising bacterium (PSB), designated as isolate PSB S-2, on the growth and yield parameters of maize (Zea mays L.). The isolate, initially screened and confirmed for its high phosphate solubilising efficiency under in vitro conditions, was selected for seed inoculation. The study comprised nine treatments arranged in a randomized block design with three replications. PSB S-2 was applied as a bioinoculant in combination with varying levels of recommended dose of fertilizers (RDF). Among the treatments, the application of PSB S-2 along with 75% RDF significantly enhanced plant growth parameters, including the number of leaves per plant and chlorophyll content. It also resulted in notable improvements in yield attributes such as the number of rows per cob, grains per row, total grains per cob, grain weight per cob, test weight, cob length, cob weight, and the number of cobs per plant. In contrast, the control treatment without any inoculant recorded the lowest values in all observed parameters. The findings suggest that PSB S-2, when combined with a reduced dose of chemical fertilizer, can effectively improve maize growth and productivity under field conditions, promoting sustainable agricultural practices.
Introduction
Maize (Zea mays L.) is one of the world’s most important cereal crops, cultivated extensively across a broad range of agro-climatic zones due to its remarkable adaptability (Shiferaw et al., 2011). It serves as a staple food in many regions and provides raw material for industrial and livestock applications. In countries like India, more than 85% of maize produced is consumed directly as human food, with grains processed into flour and incorporated into a variety of traditional dishes (Sharma and Misra, 2021). Nutritionally, maize grains are rich in carbohydrates and contain considerable amounts of vitamin A, niacin, riboflavin, and vitamin E, although the protein component, zein, is deficient in lysine and tryptophan - two essential amino acids required for human health (Vasal, 2000).
Plant nutrition, which involves the study of essential mineral elements required for plant growth and reproduction, plays a fundamental role in crop productivity. Macronutrients such as nitrogen (N), phosphorus (P), and potassium (K) are vital for various physiological processes, including cell division, photosynthesis, and energy transfer (Marschner and Marschner, 2012). Among these, phosphorus ranks next to nitrogen in importance for crop growth. It plays a critical role in nucleic acid synthesis, energy metabolism via adenosine triphosphate (ATP), and the regulation of enzymatic processes (Tisdale et al., 1993). An adequate supply of phosphorus in early plant growth stages is essential for root development and the initiation of floral primordia, ultimately influencing reproductive success (Sharma et al., 2022). However, phosphorus availability is often limited in soils due to its strong fixation with calcium, iron, and aluminum compounds, especially in alkaline and acidic soils (Hinsinger, 2001). This results in widespread phosphorus deficiency in many agricultural systems, leading to symptoms such as stunted growth, delayed maturity, and purple leaf pigmentation (Rashid et al., 2004).
To address this issue sustainably, the application of phosphate-solubilising microorganisms (PSMs) has gained attention. These include diverse bacterial and fungal genera capable of transforming insoluble phosphates like tricalcium phosphate, hydroxyapatite, and rock phosphate into bioavailable forms through acidification, chelation, and enzymatic mechanisms (Rodríguez and Fraga, 1999; Liu et al., 2020). Among these, phosphate-solubilising bacteria (PSB) such as species of Bacillus, Pseudomonas, and Rhizobium have been widely studied for their efficiency and multifunctional roles in promoting plant growth (Khan et al., 2009; Sharma et al., 2021). These bacteria not only enhance phosphorus availability but also contribute to improved nutrient uptake, biomass accumulation, and yield through the secretion of plant growth-promoting substances like indole acetic acid (IAA), siderophores, and enzymes like ACC deaminase (Vessey, 2003).
The integration of PSBs into fertilization regimes offers a promising alternative to reduce the overuse of chemical fertilizers and supports environmentally sound agricultural practices. Particularly, combining PSBs with reduced levels of chemical phosphorus fertilizers has shown potential to maintain or even enhance crop yield while minimizing environmental degradation (López-Arredondo et al., 2014; Singh and Prasanna, 2020).
The present investigation focuses on the field evaluation of an efficient phosphate-solubilising bacterial isolate (PSB S-2), which had previously demonstrated high solubilisation potential under in vitro conditions. The study aimed to assess its impact on the growth and yield attributes of maize when applied as a seed inoculant, in combination with different levels of recommended dose of fertilizers (RDF), to determine its utility in integrated nutrient management under field conditions.
Material and Methods
Experimental site and soil characteristics
A field experiment was conducted to evaluate the effectiveness of phosphate solubilising bacterial (PSB) inoculants on the growth and yield performance of maize (Zea mays L.) under black cotton soil conditions. The study was carried out at the experimental farm of the College of Agriculture, Raichur, Karnataka, India.
The experimental site is located in the northern dry zone of Karnataka, characterized by black cotton soil (Vertisol) with good moisture retention capacity.
Procurement of bacterial isolates
Two efficient PSB strains—one locally isolated and the other a standard reference strain—were obtained from the Department of Agricultural Microbiology, College of Agriculture, Raichur. These isolates had previously been screened and characterized for phosphate solubilisation potential under in vitro conditions.
Preparation of PSB inoculants
The bacterial cultures were grown in Pikovskaya’s liquid medium. The broth was prepared in 250 mL Erlenmeyer flasks, sterilized at 121°C for 30 minutes, and subsequently cooled to ambient temperature. Each flask was inoculated with 1 mL of standardized bacterial suspension (10-⁸ CFU mL⁻¹) and incubated at 37°C on a rotary shaker at 120 rpm for 72 hours. After the incubation period, the broth cultures were mixed thoroughly with sterilized lignite powder in a 1:1 ratio (w/v) to serve as a carrier material. The formulation was cured under shade for 24 hours and packed into sterile low-density polyethylene (LDPE) bags at 200 g per packet. This formulation was used as the seed treatment bioinoculant.
Seed treatment and field layout
Maize seeds (cv. Hema hybrid) were surface-sterilized using 0.1% mercuric chloride solution, rinsed with sterile distilled water, and air-dried. Treated seeds were coated uniformly with PSB inoculants using 10% jaggery solution as an adhesive.
The experiment was laid out in a randomized complete block design (RCBD) with nine treatments replicated thrice. Each treatment represented a combination of PSB inoculation and graded levels of recommended dose of fertilizer (RDF).
|
Treatments details |
|
T1 – Control |
|
T2 – PSB |
|
T3 – Reference PSB |
|
T4 – RDP 100% |
|
T5 – RDP 100% + PSB |
|
T6 – RDP 75% |
|
T7 – RDP 75% + PSB |
|
T8 – RDP 50% |
|
T9 – RDP 50% + PSB |
Growth parameter observations
Plant growth observations were recorded at 30, 60, and 90 days after sowing (DAS) and at the harvest stage to evaluate the influence of phosphate solubilising bacterial inoculants on maize development. Five plants were randomly selected and tagged in each plot for detailed measurements. The number of fully developed green leaves per plant was counted manually to assess vegetative vigor. Leaf chlorophyll concentration was measured using a SPAD-502 chlorophyll meter (Konica Minolta, Japan). For each plant, SPAD readings were taken from the uppermost fully expanded leaf at three different positions, and the average was considered as the representative value. These measurements were conducted consistently across all growth stages to monitor the photosynthetic status of the plants throughout the crop cycle.
Yield attributes and harvest observations
At the time of harvest, several yield-related parameters were assessed to determine the effect of PSB inoculation on reproductive performance and productivity of maize. The number of cobs produced per plant was recorded, followed by measurements of cob length using a standard ruler. The number of kernel rows per cob and the number of grains per row were counted to estimate grain setting efficiency, and the total number of grains per cob was calculated accordingly. Grain weight per cob was determined after manual threshing and drying to uniform moisture content. The 100-seed weight (test weight) was measured from a randomly selected grain sample and adjusted to 12% moisture content to ensure accuracy and comparability. Finally, the grain and stover yields were determined from the net plot area and extrapolated to a per hectare basis. These comprehensive yield measurements helped establish the relationship between bacterial inoculation, plant nutrition, and crop productivity under field conditions.
Results and Discussion
Effect of phosphate solubilising bacteria on number of leaves
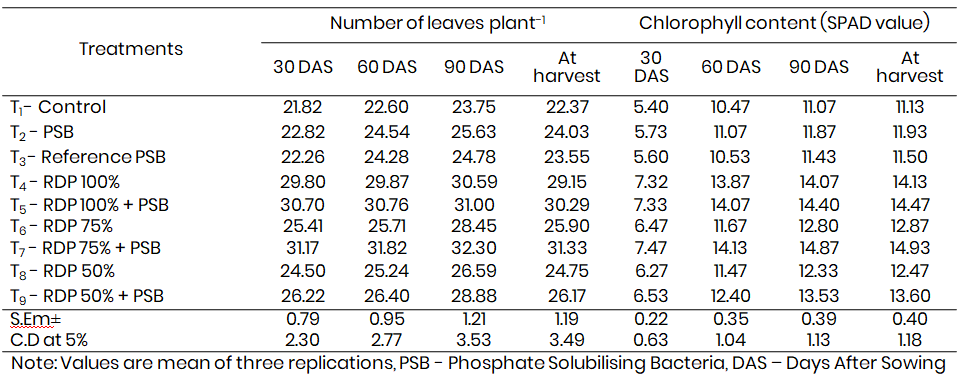
The application of efficient phosphate solubilising bacterial (PSB) isolates had a notable and statistically significant effect on the number of leaves produced by maize plants at different stages of growth. Among the treatments, the maximum number of leaves per plant was recorded at 30 days after sowing (DAS) in the treatment receiving inoculation with the efficient PSB isolate PSB-S-2 combined with 75% of the recommended dose of phosphorus (RDP), which recorded 7.47 leaves per plant. This was significantly higher than both the treatment involving inoculation with the reference PSB strain (5.60 leaves per plant) and the uninoculated control (5.40 leaves per plant). The enhanced leaf development trend continued consistently across later growth stages and was evident up to the harvest (Table 1).
This increase in leaf production can be attributed to the role of PSB in improving phosphorus availability in the rhizosphere. Phosphorus is one of the key macronutrients influencing root development and photosynthetic activity, which in turn supports enhanced vegetative growth. Ahmad et al. (2009) also observed similar improvements in plant height, number of leaves, and pod formation in chickpea due to PSB inoculation. Enhanced leaf production in cowpea plants was similarly reported by Nagaraju et al. (1995), particularly when PSB inoculants were applied in conjunction with rock phosphate (RP), leading to greater solubilisation of unavailable phosphorus forms and improved nutrient uptake. Tomar et al. (1993) also reported increased branching and foliage development due to enhanced rhizospheric P availability facilitated by P-solubilising microorganisms. According to Jat and Mali (1992), phosphorus application stimulates meristematic activity and photosynthetic efficiency, both of which are essential for leaf initiation and expansion. These earlier findings corroborate the present study’s observation that PSB-S-2, when combined with partial RDP, creates an optimal nutrient environment conducive to vigorous vegetative growth.
3.2 Chlorophyll content of maize leaves
Chlorophyll content is a direct indicator of the photosynthetic potential and physiological health of the plant. In the present investigation, the treatment involving PSB inoculation along with 75% RDP (T7) exhibited a significant increase in leaf chlorophyll concentration, especially at 60 DAS. The SPAD values in this treatment were statistically superior to those recorded in the uninoculated control and the treatment with reference PSB alone (Table 1). The improved chlorophyll content is likely the result of enhanced phosphorus availability, which plays a key role in energy transfer and photosynthesis. Phosphorus is involved in the synthesis of ATP and nucleic acids, and its availability directly influences the production of photosynthetic pigments.
These results align with the findings of Madhaiyan et al. (2004), who reported increased photosynthetic activity in plants inoculated with beneficial microbes, including PSB. Their study demonstrated that such inoculations could increase chlorophyll concentration, the number of stomata, and the accumulation of organic acids like maleic acid, which support photosynthetic function and overall plant vigor. Improved chlorophyll content in the present study suggests that PSB inoculation not only supports nutrient acquisition but also enhances physiological processes related to plant productivity.
Table 1: Influence of efficient strain of phosphate solubilising bacteria on the Number of leaves plant-1, Chlorophyll content of maize leaves at differen
Influence of PSB inoculation on yield parameters
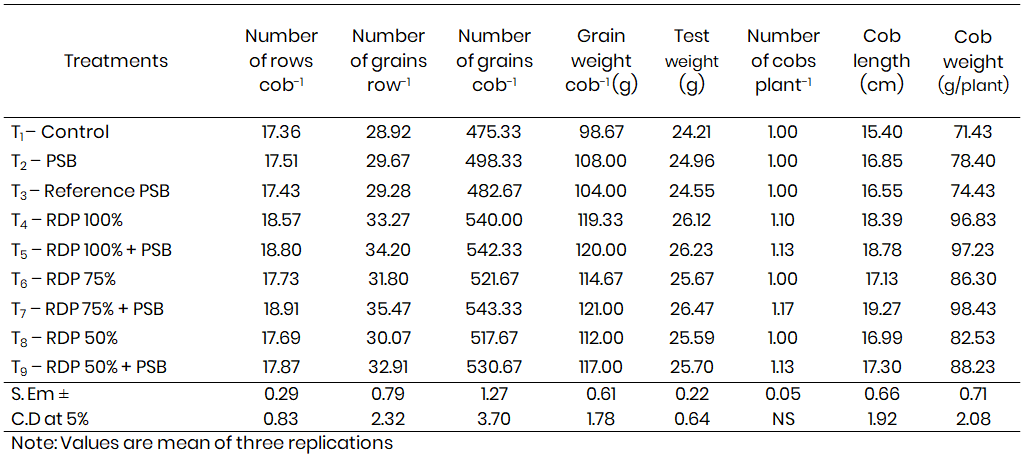
A significant improvement in yield attributes was observed due to the inoculation of the efficient PSB isolate PSB-S-2 in combination with 75% RDP. This treatment resulted in the highest values for several key yield components. The number of kernel rows per cob was 18.91, and the number of grains per row reached 35.47, resulting in an average of 543.33 grains per cob. The grain weight per cob was also enhanced (121.00 g), along with a test weight of 26.47 g. The same treatment recorded an average of 1.17 cobs per plant, cob length of 19.27 cm, and cob weight of 98.43 g per plant, all of which were significantly higher than other treatment combinations, including the uninoculated control and the treatment receiving only reference PSB.
Interestingly, although the treatment involving 100% RDP with or without PSB inoculation also performed well, it was statistically on par with T7, indicating that the combination of PSB-S-2 with 75% RDP was sufficient to match the performance of full-dose fertilizer applications. This demonstrates the potential of bioinoculants like PSB to reduce the need for chemical fertilizers while maintaining or even enhancing crop productivity.
The results are in agreement with Wu et al. (2005), who documented significant increases in maize yield and improvement in soil properties, including organic matter content, following the application of PSB. Similarly, Balsubramanian and Subramanian (2006) observed enhanced grain yield in rice due to silicate solubilising bacterial inoculation, where treated plots yielded 5218 kg ha⁻¹ compared to 4419 kg ha⁻¹ in control treatments. These findings reinforce the present study’s outcomes, highlighting that microbial inoculants not only support plant nutrition but also positively influence the soil microbiome and nutrient cycling, resulting in improved yield (Table 2).
Increased phosphorus availability from PSB activity enhances root development and nutrient uptake, which are critical during the reproductive stages of maize. The resulting improvements in cob formation, grain setting, and grain filling demonstrate the cascading benefits of microbial biofertilizers on crop performance. Furthermore, the use of PSB in combination with reduced levels of chemical fertilizers offers a sustainable and eco-friendly approach to crop production, reducing input costs and environmental impact.
Table 2: Number of rows cob-1, Number of grains row-1, Number of grains cob-1, Grain weight cob-1 and Test weight, Number
Conclusion
The present study clearly demonstrates that the inoculation of maize seeds with an efficient phosphate solubilising bacterial isolate (PSB-S-2), in combination with 75% of the recommended dose of phosphorus, significantly enhanced plant growth and yield attributes under field conditions in black cotton soil. The results indicated that this treatment notably improved critical growth parameters such as the number of leaves and chlorophyll content at various crop stages, highlighting improved vegetative vigour and photosynthetic capacity. Furthermore, yield-contributing traits including number of rows per cob, grains per cob, cob weight, test weight, and grain yield were significantly increased compared to uninoculated control and even the reference PSB strain.
The ability of PSB-S-2 to enhance phosphorus availability in the rhizosphere contributed to better nutrient uptake, which translated into improved physiological and agronomic performance. The treatment with PSB-S-2 + 75% RDP was on par with 100% RDP, suggesting that the use of efficient PSB can partially substitute chemical phosphorus fertilizers, offering a more sustainable and cost-effective alternative. These findings reinforce the potential of using microbial inoculants like PSB as a viable component of integrated nutrient management strategies for maize, ensuring higher productivity with reduced dependence on synthetic fertilizers while maintaining soil health and environmental sustainability.
References
Ahmad, F., Ahmad, I., & Khan, M. S. (2009). Screening of free-living rhizospheric bacteria for their multiple plant growth promoting activities. Microbiological Research, 164(2), 173–181.
Balsubramanian, A., & Subramanian, S. (2006). Effect of silicate solubilising bacteria on yield and uptake of rice under field conditions. Agricultural Science Digest, 26(3), 167–170.
Hinsinger, P. (2001). Bioavailability of soil inorganic P in the rhizosphere as affected by root-induced chemical changes: A review. Plant and Soil, 237(2), 173–195. https://doi.org/10.1023/A:1013351617532
Jat, R. L., & Mali, A. L. (1992). Effect of phosphorus and zinc fertilization on growth and yield of cowpea. Indian Journal of Agronomy, 37(4), 861–863.
Khan, M. S., Zaidi, A., & Wani, P. A. (2009). Role of phosphate-solubilizing microorganisms in sustainable agriculture – A review. Agronomy for Sustainable Development, 29(1), 43–54. https://doi.org/10.1051/agro:2008024
Liu, H., Wu, Y., Wang, S., & Qiao, J. (2020). Application of phosphate solubilizing bacteria in sustainable agriculture: Benefits and challenges. Journal of Environmental Management, 260, 110074. https://doi.org/10.1016/j.jenvman.2019.110074
López-Arredondo, D. L., Leyva-González, M. A., González-Morales, S. I., López-Bucio, J., & Herrera-Estrella, L. (2014). Phosphate nutrition: Improving low-phosphate tolerance in crops. Annual Review of Plant Biology, 65, 95–123. https://doi.org/10.1146/annurev-arplant-050213-035949
Madhaiyan, M., Poonguzhali, S., Ryu, J., & Sa, T. (2004). Regulation of ethylene levels in canola (Brassica campestris) by 1-aminocyclopropane-1-carboxylate deaminase containing Methylobacterium fujisawaense. Planta, 220, 497–505.
Marschner, P., & Marschner, H. (2012). Marschner’s mineral nutrition of higher plants (3rd ed.). Academic Press.
Nagaraju, V., Reddy, D. D., & Reddy, G. (1995). Rock phosphate dissolution and P availability as influenced by phosphobacteria and VAM fungi in an Alfisol. Journal of the Indian Society of Soil Science, 43(1), 118–122.
Rashid, A., Ryan, J., & Memon, M. (2004). Phosphorus use efficiency in soils and crops. In Nutrient management for sustainable crop production in Asia (pp. 159–187). IFDC
Rodríguez, H., & Fraga, R. (1999). Phosphate solubilizing bacteria and their role in plant growth promotion. Biotechnology Advances, 17(4–5), 319–339. https://doi.org/10.1016/S0734-9750(99)00014-2
Sharma, A., Singh, R., & Kumari, B. (2021). Effect of phosphate-solubilizing bacteria on soil properties and maize productivity. Journal of Soil Biology and Ecology, 41(2), 95–102.
Sharma, R., & Misra, S. (2021). Nutritional and economic importance of maize cultivation in rural India. Journal of Agricultural Economics and Development, 10(1), 12–18.
Sharma, V., Meena, R. S., & Ghosh, P. K. (2022). Role of phosphorus nutrition in crop growth and productivity: An overview. International Journal of Plant and Soil Science, 34(24), 75–83.
Shiferaw, B., Prasanna, B. M., Hellin, J., & Bänziger, M. (2011). Crops that feed the world 6. Past successes and future challenges to the role played by maize in global food security. Food Security, 3, 307–327. https://doi.org/10.1007/s12571-011-0140-5
Singh, R., & Prasanna, R. (2020). Phosphorus biofertilizers: An eco-friendly approach to improve phosphorus use efficiency. Ecological Indicators, 110, 105881. https://doi.org/10.1016/j.ecolind.2019.105881
Tisdale, S. L., Nelson, W. L., Beaton, J. D., & Havlin, J. L. (1993). Soil fertility and fertilizers (5th ed.). Macmillan Publishing Company.
Tomar, M. S., Namdeo, K. N., & Deshmukh, M. R. (1993). Effect of phosphate solubilizing organisms on the yield and nutrient uptake of moong. Journal of Soils and Crops, 3(1), 65–67.
Vasal, S. K. (2000). The quality protein maize story. Food and Nutrition Bulletin, 21(4), 445–450. https://doi.org/10.1177/156482650002100412
Vessey, J. K. (2003). Plant growth promoting rhizobacteria as biofertilizers. Plant and Soil, 255, 571–586. https://doi.org/10.1023/A:1026037216893
Wu, S. C., Cao, Z. H., Li, Z. G., Cheung, K. C., & Wong, M. H. (2005). Effects of biofertilizer containing N-fixer, P and K solubilizers and AM fungi on maize growth: A greenhouse trial. Geoderma, 125(1–2), 155–166.
Copyright
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.

